3950
Evaluating Universal and Fast Online Customized Pulses for parallel transmission using two different RF coils1Institue of Neuroradiology, University Hospital Erlangen, Erlangen, Germany, 2Imaging Centre of Excellence, University of Glasgow, Glasgow, Scotland, 3SIEMENS Healthineers, Erlangen, Germany, 4Institute of Neuroscience & Psychology, University of Glasgow, Glasgow, Scotland, 5MR CoilTech Limited, Glasgow, Scotland, 6Institue of Radiology, University Hospital Erlangen, Erlangen, Germany, 7Friedrich Alexander University Erlangen Nürnberg, Erlangen, Germany
Synopsis
To investigate the universality of using pre-optimized parallel-transmit (pTx) pulses with different RF coils for routine imaging, Universal Pulses and Fast Online Customized Pulses were generated using eight respective datasets from both a commercial and a self-built 8Tx/32Rx coil. The pTx pulse types were designed for both excitation and inversion pulses in a 3D MPRAGE sequence. For each coil, one subject was examined with all combinations of pTx pulses. All pTx pulses outperform CP pulses on the coil they were trained on. When using a different coil, Universal Pulses may fail, especially for inversion, whereas FOCUS pulses achieve robust homogeneity.
MRI at 7 tesla is strongly affected by inhomogeneous B1 fields causing signal variations throughout the brain, which can be mitigated with parallel transmit (pTx). For clinical application, either predefined pTx Universal Pulses (UPs) [1, 2] (1) or Fast Online Customized (FOCUS) pulses [3] (2) are desirable. Here we investigate the general usability of these two pulse types with one commercial and one self-built 8Tx/32Rx RF head coil [4]. Four non-selective pTx pulses were designed for each coil, consisting of UP/FOCUS excitation/inversion pulses for use in an MPRAGE sequence.
Methods
All measurements were performed on two 7T whole-body MR systems (Magnetom Terra, Siemens Healthcare, Erlangen, Germany) one using the self-built and one the commercial coil (Nova Medical, Wilmington, USA). From each system, previously acquired datasets (B1+/B0 maps) were used for optimizing pTx pulses.
The excitation pulses used an extended SPINS k-space trajectory [3, 5], defined by the parameters PSPINS, had a target flip angle (FA) of 5° and a fixed duration of 1ms. The inversion pulses used a kT point trajectory (7 kT points, first one at the k-space centre) [6] and a maximum duration of 4.8ms. Parameters defining the trajectory PkT consisted of the Cartesian coordinates of the nonzero kT points and the respective subpulse durations. The trajectory parameters, combined with energy regularization weights λ, were defined as ‘Combined Optimization Values’ (COV) for offline optimization:
$$COV_{Exc} ∶= [P_{SPINS},λ] $$
$$COV_{Inv} ∶= [P_{kT},λ] $$
For each set of COV, individual pulse shape optimization and a Bloch simulation were performed for all datasets. The returned subject specific values for normalized root mean square error (NRMSEp) of the FA and maximum local specific energy dose (SEDp) in [J/kg] were used for the cost function shown below [3]. SEDp was determined by summing SAR values over the pulse using the coil specific virtual observation point (VOP) models [7].
$$min∑_{p=1}^{N_p} [w_{Hom} exp(NRMSE_p (COV_{Exc/Inv}))+ w_{SED} exp(SED_p(COV_{Exc/Inv}))] $$
This approach used empirically determined weights wHom for homogeneity and wSED for SAR ([wHom, wSED ]=[5,1] for excitation, [wHom, wSED ]=[100,1] for inversion pulses).
After an optimized set of COV was found, corresponding universal pulse shapes were generated with B1+/B0 maps of all respective subjects using the Variable Exchange algorithm [8] for the excitation pulses (98 variable coefficients per channel, calculated RF shapes only) and a customized interior-point algorithm [9] for the inversion pulses (7 variable coefficients per channel, recalculated kT point locations and subpulse durations). FOCUS pulses were calculated using these algorithms and corresponding COV/UPs in maximum 45s. The generated pulses for the self-built/commercial coil and for excitation/inversion are denoted accordingly: ‘UP/FOCUSinv/exc, sb/com’.
When applying FOCUS pulses, B1+ and B0 mapping was performed during the sequence preparation phase. B1+ mapping was done using a magnetization-prepared, saturation-recovery TurboFLASH sequence (TAq = 40s) [10]. B0 mapping was performed with a sagittally oriented gradient echo sequence (TE1 = 2.39s, TE2 = 4.59s, TE3 = 7.09s; 4x4x6mm3; TAq = 12s). A mask was generated cutting off low intensity voxels. The pTx pulses were used in a 3D-MPRAGE sequence (TR = 3s, TI = 1.1s, TE = 2.28ms, FoV = 250x224x160mm3, 1mm3 iso, TAq =4min 54s). B0 shimming was performed in all measurements.
At each site, nine MPRAGE scans were performed in a different healthy subject. Five MPRAGE images were acquired using the UP designed for the respective coil for excitation and either UP/FOCUSinv sb/com or an standard adiabatic pulse for inversion. Four additional MPRAGE images were acquired using the other UP/FOCUSexc sb/com pTx pulses or a CP pulse for excitation and the same adiabatic hyperbolic secant pulse for inversion.
Results and Discussion
Figure 1 shows NRMSE, energy and SED values of all excitation pulses evaluated on all datasets compared to CP mode excitation. UPs outperform the CP excitation for their respective coils, but may result in high NRMSE values for the other (nonbrain tissue included). FOCUS pulses generally reach lower NRMSE values. The self-built coil’s VOP model produces lower SED estimates than the other and here UP/FOCUSsb have higher energy but lower SED than the CP pulse.
Figure 2 shows the same of all inversion pulses. The advantage of FOCUS compared to UPs is generally smaller than for excitation pulses, which could be related to less degrees of freedom (less variable RF coefficients per channel).
Figure 3 shows FA maps and MPRAGE images for all excitation pulses on both volunteers (same inversion pulse). UPs generally show better homogeneity than CP pulses, both FOCUS pulses generally further improve homogeneity.
Figure 4 shows FA maps and MPRAGE images for all inversion pulses (coil-specific UP excitation pulse). UPinv, sb shows severe, UPinv, com and FOCUSinv, sb show minor imperfections on the commercial coil. On the self-built coil, minor imperfections are visible for UP/FOCUSinv, sb.
Conclusion
For excitation, UPs provide better brain coverage than CP pulses, even when trained on a different coil with similar configuration yet different VOP models. Inversion pulses, however, may fail in that case. FOCUS pulses outperform both UPs also when trained on a different coil and thereby demonstrate a more general usability. FOCUS pTx inversion pulses may serve as a low-SAR alternative to adiabatic inversion pulses.
Acknowledgements
No acknowledgement found.References
1. Gras, V., et al., Universal pulses: A new concept for calibration-free parallel transmission. Magn Reson Med, 2017. 77(2): p. 635-643.
2. Gras, V., et al., Homogeneous non-selective and slice-selective parallel-transmit excitations at 7 Tesla with universal pulses: A validation study on two commercial RF coils. PLoS One, 2017. 12(8): p. e0183562.
3. Herrler, J., et al., Fast Online Customized (FOCUS) Parallel Transmission Pulses: A Combination of Universal Pulses and Individual Optimization. Magnetic Resonance in Medicine, 2020.
4. Gunamony, S., et al. An 8-channel transmit 32-channel receive 7T head coil for 1Tx and pTx scanner modes. in ISMRM. 2019. Canada.
5. Malik, S.J., et al., Tailored excitation in 3D with spiral nonselective (SPINS) RF pulses. Magn Reson Med, 2012. 67(5): p. 1303-15.
6. Cloos, M.A., et al., kT -points: short three-dimensional tailored RF pulses for flip-angle homogenization over an extended volume. Magn Reson Med, 2012. 67(1): p. 72-80.
7. Eichfelder, G. and M. Gebhardt, Local Specific Absorption Rate Control for Parallel Transmission by Virtual Observation Points. Magnetic Resonance in Medicine, 2011. 66(5): p. 1468-1476.
8. Setsompop, K., et al., Magnitude least squares optimization for parallel radio frequency excitation design demonstrated at 7 Tesla with eight channels. Magn Reson Med, 2008. 59(4): p. 908-15.
9. Majewski, K. and D. Ritter, First and second order derivatives for optimizing parallel RF excitation waveforms. J Magn Reson, 2015. 258: p. 65-80.
10. Fautz, H.-P., et al. B1 mapping of coil arrays for parallel transmission. in ISMRM 16. 2008. Toronto.
Figures
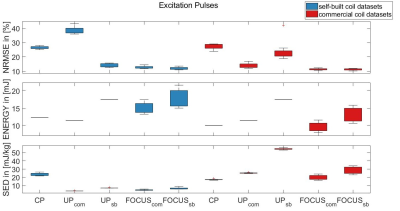
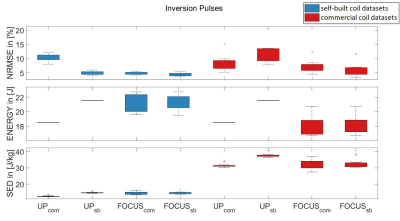
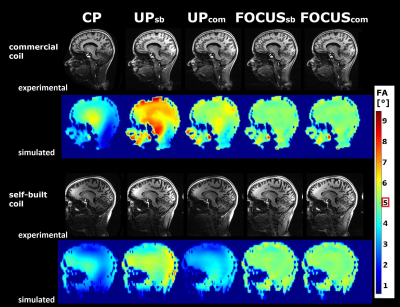
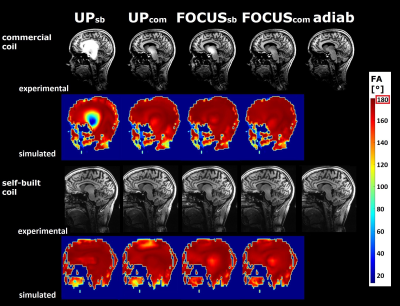
Figure 4 MPRAGE images using the UP of the respective coil for excitation and various inversion pulses. Experimental images and corresponding FA simulations are shown for coil-specific CP pulses and all generated pTx excitation pulses.