3947
Respiration induced B1+ changes and its compensation via respiration robust 3D kT point pulses in 7T body imaging1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 2Division of Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom, 3Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany
Synopsis
We demonstrate the design and application of respiration-specific and respiration-robust three-dimensional 4kT-point pTx pulses using respiration-resolved 3D B1+ maps. The subject-specific pulses were tested on 20 B1+ maps (shallow/deep breathing) of 10 volunteers with different age and BMI and were experimentally validated in the last three volunteers at 7T. Compared to respiration-specific pulses, respiration-robust pulses resulted in a negligible overall decrease of the FA homogeneity with clear benefits of achieving homogeneous 3D FA across all respiration states.
Purpose
Parallel transmission (pTx)1 in the human body is typically done for each subject individually and thus, requires subject-specific B1+ information2,3. For two-dimensional cardiac breath-hold imaging, however, a recent study showed severe B1+ changes between deep inhale and exhale impacting conventional slice-selective pTx spoke pulses. This impact was avoided by generating respiration-robust spokes using breath-hold acquired B1+ maps from multiple respiration-states4. However, the problems further increase for three-dimensional (3D) body imaging, which is investigated in detail and successfully compensated in the present work in multiple ways: First, we extend previous 3D kT-point body pulses3 towards respiration-specific pulses using respiration-resolved (RR) 3D B1+ maps2. Second, we investigate the use of RR and non-respiration-resolved (NRR) B1+ maps for shallow and deep breathing. Finally, we apply the aforementioned respiration-robust pulse-design principle4 to 3D respiration-robust kT-point pulses and perform comprehensive tests on 20 B1+ maps (shallow/deep breathing) of 10 volunteers with different age and BMI. The optimized pulses were experimentally validated in the last three volunteers at 7T.Methods
MRI was performed at 7T (Siemens Magnetom, Germany) according to an approved IRB protocol in 10 healthy volunteers (4M/6F, mean:31y, range:24-56y) with varying BMI (mean:23.5kg/m2, range:21-28kg/m2) using a certified 32-element body coil array (MRI.TOOLS, Germany) driven in 8TX/32RX mode. Local/global SAR limits in first level mode (IEC60601-2-33) were complied by limiting each transmit channel's RF power. Relative 3D B1+-maps of the thorax were acquired for each volunteer under free breathing2 with a radial phase-encoding trajectory5 (nominal FA=20°, TE/TR=2.02/40ms, FOV=250x312x312mm3, resolution = (4mm)3). All volunteers were instructed via the intercom to perform deep breathing patterns and to breath regularly otherwise. 256/512 RPE-lines were acquired in 205s/410s for shallow/deep breathing, respectively. The B1+ maps were reconstructed NRR and RR for three (shallow) and five (deep) respiration states2. Subject-specific 4kT-point pulses were designed using the small-tip-angle approximation in MATLAB for the manually selected NRR and RR 3D heart volumes to optimize respiration-specific and respiration-robust 4kT-point pulses. The 4kT-point pulses (duration=0.96ms) were computed in <30s online using an automatic regularization parameter update that balances the root-mean-squared FA error in the heart ROI and the total RF power3,6. The pulse performance was measured by the $$$\text{CV}=std(\text{FA})/mean(\text{FA})$$$ in the ROIs. High-resolution 3D gradient-echo scans (nominal FA=10°, TE/TR=1.75/3.7ms, FOV=250x312x312mm3, resolution=(1.4mm)3, 256 RPE-lines, TA=333s) have been acquired in three volunteers to validate the respiration-robust 4kT-point pulses.Results and Discussion
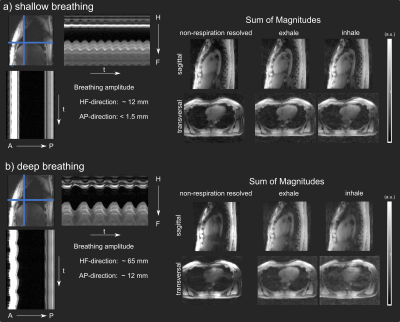
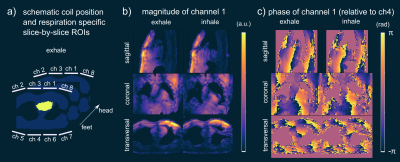
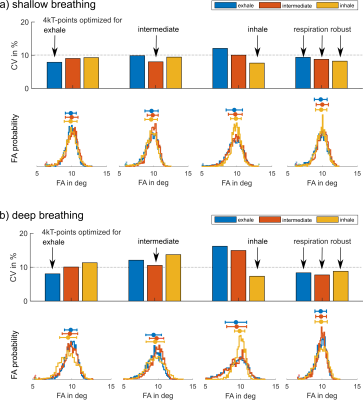
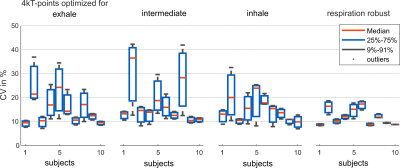
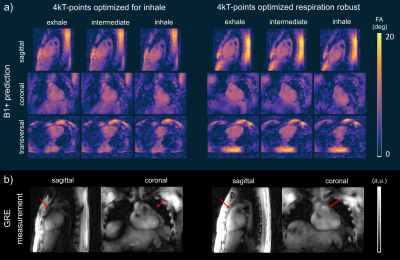
Fig.1 illustrates the breathing amplitude of shallow (a) and deep (b) breathing and the impact of respiratory motion on the 3D GRE images from NRR and two RR reconstructions (inhale and exhale). Shallow breathing primarily results here in similar NRR and RR reconstructions. Because more data is available for the reconstruction, NRR reconstructions are preferable due to an increased signal-to-noise (SNR) ratio and reduced artifacts. For deep breathing, however, we see a clear difference between NRR and RR reconstructions as a result of strong respiratory motion (HF~65mm, AP~12mm). Here, the use of RR reconstructions and, thus, the use of respiration-specific or respiration-robust pTx pulses for stronger breathing patterns is preferable. Fig.2a shows one slice of the 3D heart ROI and the schematic coil position. ROIs were selected manually for each respiration state. Fig2.b-c show the relative B1+ magnitude and phase (relative to channel 4) of one out of 8 channels (ch1) with a clear difference between the exhale and the inhale state. Fig.3 shows the resulting CVs in the heart ROIs of subject 7 (exhale, intermediate and inhale state) for shallow (a) and deep (b) breathing. Depicted are four optimization settings: exhale, intermediate, inhale and respiration-robust. As expected, only small CV differences were observed for shallow breathing (mean=9%, range=8-12%) with comparable FA distributions, thus supporting the use of NRR reconstructions in the case of shallow breathing3. For deep breathing, however, the CV differences were more pronounced (mean=10%, range=7-17%). For instance, the pulse optimized for inhale (CV=7%) resulted in a CV of 17% for exhale. As expected, the respiration-robust optimization decreased the FA spread and even more remarkably, the CVs of the respiration-robust pulse (8-9%) remained in the same range as the CVs of the respiration-specific results (8-10%). Fig.4. summarizes the CVs of all 10 volunteers for the respiration-specific and respiration-robust pulses for deep breathing patterns. Across all subjects, the 3D respiration-robust pulses achieved mean CVs ranging from 8.5% (subject 1) to 16.9% (subject 6). This is much lower compared to respiration-specific pulses with mean CVs ranging from 9% (subject 1) to unacceptable 36.3% (subject 2). Fig.4a shows the 3D B1+ prediction using the subject-specific inhale (left) and the respiration-robust (right) 4kT-point pulse. Fig.4b shows the acquired, 3D GRE images (acquired without cardiac gating) using the same pulses. The 3D GRE images are reconstructed for the exhale state to demonstrate the advantages of the respiration-robust pulse.Conclusion
While NRR B1+ maps are suitable for 7T 3D cardiac MR imaging under shallow breathing, this in-vivo study demonstrates that RR maps and respiration-robust 4kT pTx pulses are highly preferred to achieve 3D heart FA homogenization at 7T when subjects perform strong breathing. Compared to respiration-specific pulses, respiration-robust pulses resulted in a negligible overall decrease of the FA homogeneity with clear benefits of achieving homogeneous 3D FA across all respiration states.Acknowledgements
We gratefully acknowledge funding from the German Research Foundation SCHM 2677/2-1 and GRK2260, BIOQIC.References
1) Padormo, F., Beqiri, A., Hajnal, J. V., and Malik, S. J. (2016) Parallel transmission for ultrahigh‐field imaging. NMR Biomed., 29: 1145– 1161. doi: 10.1002/nbm.3313
2) Dietrich, S., Aigner, C.S., Kolbitsch, C., et al. (2020) 3D Free-breathing Multi-channel absolute B1+ Mapping in the Human Body at 7T, Magn. Reson. Med., doi:10.1002/mrm.28602
3) Aigner, C.S., Dietrich, S. and Schmitter, S. (2020) Three-dimensional static and dynamic parallel transmission of the human heart at 7T, NMR in Biomed., doi:10.1002/nbm.4450
4) Schmitter, S., Wu, X., Uğurbil, K., Van de Moortele, PF. (2015) Design of parallel transmission radiofrequency pulses robust against respiration in cardiac MRI at 7 Tesla. Magn. Reson. Med., 74 doi: 10.1002/mrm.25512
5) Prieto, C., Uribe, S., Razavi, R., Atkinson, D. and Schaeffter, T., (2010) 3D undersampled golden‐radial phase encoding for DCE‐MRA using inherently regularized iterative SENSE. Magn. Reson. Med., 64, pp. 514-526, doi:10.1002/mrm.22446
6) Cao, Z., Yan, X. and Grissom, W.A. (2016) Array‐compressed parallel transmit pulse design. Magn. Reson. Med., 76, pp. 1158-1169., doi:10.1002/mrm.26020
Figures