3945
Diagnostic Consensus in the Interpretation of Ultra-High-Field MRS in Glioma Patients1School of Health Sciences, Purdue University, West Lafayette, IN, United States, 2Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN, United States, 3Radiology, University Medical Centre Utrecht, Utrecht, Netherlands, 4University of Oxford, Oxford, United Kingdom, 5Antoni van Leeuwenhoek Hospital, Netherlands Cancer Center, Amsterdam, Netherlands, 6Brigham and Women’s Hospital / Dana Farber Cancer Institute, Harvard Medical School, Boston, MA, United States, 7Department of Neurosurgery, University of Minnesota, Minneapolis, MN, United States
Synopsis
We have recently initiated a 7T MRS glioma consortium, intending to bring together experts in the field to discuss pitfalls, promises, and potential research avenues of MRS in gliomas. During the "GlioMaRS-NET Workshop" in 2020, we tested the efficacy of UHF MRS for predicting the molecular characteristics by visual inspection by conducting a survey with a set of previously acquired UHF spectra from glioma patients. There was a high concordance in diagnostic interpretation of spectra for the identification of IDH-mutant gliomas versus IDH-wildtype gliomas.
Introduction
The World Health Organization (WHO) classification of gliomas relies on integrated histomolecular diagnostics. This offers prospects for precision medicine strategies tailoring therapies for each individual. The International Society of Neuropathology‐Haarlem Consensus has proposed a personalized “layered” diagnostic approach for gliomas that integrates relevant data acquired through different modalities. he final diagnosis (Layer 1), relies on all available data, which include the histological classification (Layer 2, e.g., astrocytoma vs. oligodendroglioma), the grading (Layer 3, e.g., WHO grade II vs. III), and molecular characteristics (Layer 4, e.g., isocitrate dehydrogenase (IDH)-mutant, 1p/19q-codeleted) (1). Here we argue that non-invasive “molecular surrogate” markers acquired through MRS could contribute to this approach.It has recently been shown that definitional features of glioma, such as IDH mutation and 1p/19q codeletion (Layer 4), can be identified with non-invasive MRS at 3T, opening up exciting new opportunities for diagnostics, clinical trials, and assessment of treatments (2,3). Ultra-high-field (UHF, >=7T) MRI scanners offer enhanced detection relative to routine 3T MRI of 2-HG peaks in the MRS spectra of IDH mutated patients (4,5). This is due to increased SNR at the higher field strength and increased spectral dispersion, which can improve the delineation of 2HG from neighboring metabolites such as glutamate and glutamine. Furthermore, at 7T, the 2-HG peak is not only to contribute to layer 4 of the integrated diagnosis by identifying molecular features but also to detect subtle changes due to the reprogramming of cellular metabolism during the disease progression or treatment.
With the recent successes of 2-HG identification and increased use of UHF MRI in clinical settings, we have recently initiated a 7T MRS glioma consortium, intending to bring together experts in the field to discuss pitfalls, promises, and potential research avenues of MRS in gliomas. During the "Multi-center 7T Glioma Consortium (GlioMaRS-NET) Workshop" held on 16-20 November 2020 (https://www.ndcn.ox.ac.uk/study-with-us/continuing-professional-development/gliomars ), we tested the diagnostic consensus for visual inspection of UHF MRS spectra for predicting the molecular characteristics of gliomas using previously acquired UHF spectra.
Methods
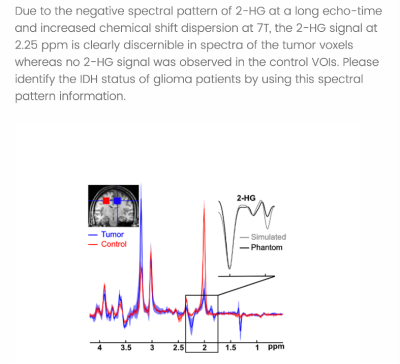
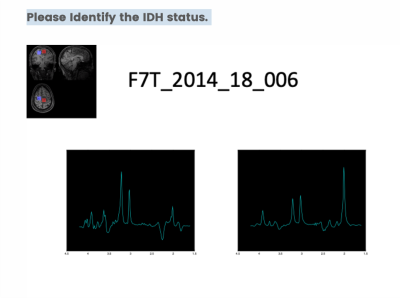
For this purpose, we asked the attendees of the first GlioMaRS-NET workshop to complete a survey in which participants predicted the patients' IDH status by inspecting the UHF MRS spectra visually. A total of 13 glioma patients' 7T MRS single-voxel MRS spectra were used for the survey. Assessed by immunohistochemical and DNA sequencing from a surgical tissue biopsy, nine patients were identified carrying IDH mutations. In the survey, MRS voxel placements on each patient's T1-weighted anatomical MPRAGE were provided. The 2-HG sensitive semi-localization by adiabatic selective refocusing (semi-LASER, TE=110 ms) pulse sequence spectra acquired at 7T were illustrated for voxel of interest in the tumor tissue and if available in the healthy tissue (5). Only preprocessed spectra (frequency and phase-corrected) were shown, without any information on metabolite fits or quantification. Figure 1 illustrates the information provided to participants at the beginning of the survey. Figure 2 shows the representative question asked for the participants to predict the IDH status by visual inspection of the spectra.Results
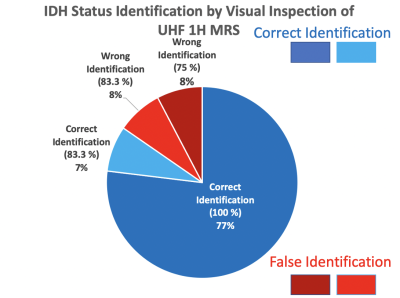
A total of 12 participants consisting of neuro-oncologists, neurosurgeons, biochemists, pathologists, and MR physicists answered the questionnaire during the GlioMaRS-NET workshop (https://purdue.ca1.qualtrics.com/jfe/form/SV_3t0rhgTgMqzxAuV). Descriptive measures of the predicted IDH status from the visual inspection are shown in Figure 3. All participants identified 11 glioma patient IDH statuses agreed with clinical molecular statuses by visual inspection with an accuracy range between 83.3-100 %. In other words, all participants identified the IDH statuses of 10 patients successfully. Only two glioma patients' IDH statuses were identified falsely with a range of between 83.3 (10 participants (False) vs. 2 participants) and 75 % (9 participants (False) vs. 3 participants).Conclusion and Discussion
Several studies have demonstrated in vivo detection of 2-HG in IDH-mutant tumors at 3T, commonly used in the clinical setting (2,6)(7)(8). However, the assignment of 2-HG resonances at 3T is a technical challenge not only because of the complex spin-coupling features of overlapping resonances but also due to the lack of SNR and spectral resolution. As we demonstrate, the IDH status could be detected by simple visual inspection of spectra acquired at 7T in the absence of any other information. The performance is expected to increase with a better-powered study and the use of machine learning. The collection of prospectively acquired UHF MRS spectra from multiple international centers could lead to a widely accepted prognostic precision medicine biomarker detection system for identifying, stratifying, and monitoring IDH1 and IDH2 mutant glioma patients.Acknowledgements
No acknowledgement found.References
1. Louis DN, Perry A, Burger P, et al. International Society of Neuropathology-Haarlem Consensus Guidelines for Nervous System Tumor Classification and Grading. Brain Pathology 2014;24:429–435 doi: https://doi.org/10.1111/bpa.12171.
2. Choi C, Ganji SK, DeBerardinis RJ, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated glioma patients. Nat Med 2012;18:624–629 doi: 10.1038/nm.2682.
3. Branzoli F, Deelchand DK, Sanson M, Lehéricy S, Marjańska M. In vivo 1H MRS detection of cystathionine in human brain tumors. Magnetic Resonance in Medicine 2019;82:1259–1265 doi: https://doi.org/10.1002/mrm.27810.
4. Berrington A, Voets NL, Larkin SJ, et al. A comparison of 2-hydroxyglutarate detection at 3 and 7 T with long-TE semi-LASER. NMR Biomed 2018;31 doi: 10.1002/nbm.3886.
5. Emir UE, Larkin SJ, de Pennington N, et al. Noninvasive quantification of 2-hydroxyglutarate in human gliomas with IDH1 and IDH2 mutations. Cancer research 2016;76:43–49.
6. Andronesi OC, Kim G, Gerstner E, et al. Detection of 2-Hydroxyglutarate in IDH-mutated Glioma Patients by Spectral-editing and 2D Correlation Magnetic Resonance Spectroscopy. Sci Transl Med 2012;4:116ra4 doi: 10.1126/scitranslmed.3002693.
7. Berrington A, Voets NL, Plaha P, et al. Improved Localization for 2-Hydroxyglutarate Detection at 3 T Using Long-TE Semi-LASER. Tomography 2016;2:94–105 doi: 10.18383/j.tom.2016.00139.
8. Pope WB, Prins RM, Thomas MA, et al. Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J Neurooncol 2012;107:197–205 doi: 10.1007/s11060-011-0737-8.