3939
Development and optimization of diffusion-weighted imaging protocols for the optic nerve and pituitary gland
Zhiqiang Li1, Sharmeen Maze1, and John P Karis1
1Neuroradiology, Barrow Neurological Institute, Phoenix, AZ, United States
1Neuroradiology, Barrow Neurological Institute, Phoenix, AZ, United States
Synopsis
DWI helps reveal many neurological disorders. However, its application in the optic nerve and pituitary gland is hindered by strong distortion artifacts associated with ssEPI, the method of choice for clinical DWI. Recently, IRIS (an msEPI sequence) and SPLICE-PROPELLER (a distortion-free technique) have become available in the clinic. In this work, we develop and optimize DWI protocols for imaging the optic nerve and pituitary gland. Volunteer studies and patient scans demonstrate immunity to geometric distortions, and good overall image quality with clinically friendly scan times.
Introduction
Diffusion-weighted MRI (DWI) is an invaluable tool for the diagnosis of many neurological disorders. However, its application outside the brain has been significantly hindered by the strong geometric distortion artifacts associated with single-shot echo planar imaging (ssEPI), which is the method of choice in the clinic for DWI due to its scan efficiency. These geometric distortion artifacts have been reduced with a variety of improvements including multi-shot EPI (msEPI)1-3 and reduced field of view imaging (rFOV)4-6. Nevertheless, residues may still be present in areas with strong field inhomogeneities or tissue susceptibilities, which are particularly problematic in the skull base. A distinct group of turbo-spin-echo-based (TSE) techniques are free from geometric distortion artifacts, but typically suffer from low scan efficiency. Therefore, DWI in two important clinical applications, the optic nerve7,8 and the pituitary gland9,10, has not been widely adopted. Recently, two new product sequences have been released and become available in the clinic: a multi-shot EPI (IRIS)3 technique, and a TSE-based technique11,12 with PROPELLER acquisition (periodically rotated overlapping parallel lines with enhanced reconstruction)13 and a SPLICE signal pathway (split echo acquisition of fast spin-echo signals)14. In this project, we aim to develop and optimize DWI protocols for the optic nerve and pituitary gland in the clinic environment, and validate the performance in volunteer and patient scans.Methods
The development was performed on a Philips 3T Ingenia scanner with a 15-channel phased-array coil. Two recently released product DWI techniques, msEPI (IRIS)3 and SPLICE-PROPELLER11, were first compared in terms of geometric distortion artifacts in the optic nerve and pituitary gland. msEPI was dropped from the list because of the residual distortion artifacts in both anatomies. In the second stage, options of SPLICE-PROPELLER, such as voxel size, echo train length (ETL), fat suppression approaches, view ordering, gradient mode, etc., were further tuned to produce the best overall quality in the clinical setting. These parameters were adjusted to balance the scan time, image sharpness, and signal-to-noise ratio (SNR), to achieve the best diagnostic value. A few comparisons were reported in the Results section. A neuroradiologist reviewed the results and picked the set with best overall quality. The optimal imaging parameters include: field of view = 220x220 mm2 for optic nerve and 240x240 mm2 for pituitary gland, resolution = 1.5x1.5 mm2, thickness = 4 mm, recon resolution = 512x512, ETL = 18, TR = 2 s, b = 0 and 1000 s/mm2, linear view ordering, enhanced gradient mode (with maximum gradient strength), and fat suppression with and without gradient reversal for the optic nerve and pituitary gland protocols, respectively, scan time = 4:04. The proposed protocols were tested with volunteer studies, and validated in patient scans.Results and Discussion
The immunity of SPLICE-PROPELLER to geometric distortion artifacts are illustrated in Figure 1 and compared to msEPI (IRIS). The severe distortion or the signal pileup in the optic nerve and pituitary gland typically seen in ssEPI (not shown) is reduced in msEPI, but remaining residues still make the diagnosis challenging and often render the images non-diagnostic. Successfully eliminating these artifacts, SPLCE-PROPELLER becomes the option for DWI of the optical nerve and pituitary gland. In this comparison, msEPI was scanned with similar parameters, EPI echo train = 19~21, scan time = 3:56.Figure 2 compares the selected optimal set of imaging parameters of SPLICE-PROPELLER with three other options, one at a time. An enhanced gradient mode that reduces the echo time and therefore higher SNR is chosen (vs. a max mode). A linear view order is selected as the low-high (or center-out) view order generates higher SNR but does introduce blurriness as well as some non-uniformity. In addition to SPIR (spectral presaturation with inversion recovery), gradient reversal is used to help further suppress fat signals around the optic nerve. However, gradient reversal is not employed for imaging the pituitary gland as it may lose some fine structures. Without gradient reversal, the pituitary gland may also stand out better from the background in the ADC map, which is often used as a quantitative measure in this application.
Figure 3 demonstrates the performance of SPLICE-PROPELLER with the selected parameters in volunteers. These images exhibit good overall quality that meets the expectation of neuroradiologists at our institution.
Presented in Figure 4 are exemplary results of patients with indications in the pituitary gland. As observed, consistent image quality is achieved.
Conclusion
In summary, SPLICE-PROPELLER-based DWI protocols have been developed and optimized for the optic nerve and pituitary gland, two clinical applications that have not been widely explored due to the limitation of current EPI-based DWI techniques. The proposed protocols produce distortion-free images with balanced image quality and scan time. The performance has been validated in volunteer studies and patient examinations in the clinical settings.Acknowledgements
We thank Philips Healthcare for providing temporary product keys for clinical studies.References
- Holdsworth SJ, Skare S, Newbould RD, Guzmann R, Blevins NH, Bammer R. Readout-segmented EPI for rapid high resolution diffusion imaging at 3T. Eur J Radiol. 2008;65:36-46. PMCID: PMC3360876
- Chen N-k, Guidon A, Chang H-C, Song AW. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimage. 2013;72:41-47. PMCID: PMC3602151
- Jeong H-K, Gore JC, Anderson AW. High-resolution human diffusion tensor imaging using 2-D navigated multishot SENSE EPI at 7 T. Magn Reson Med. 2013;69:793-802. PMCID: PMC3424313
- Symms MR, Wheeler-Kingshott CA, Parker GJM, Barker GJ. Zonally-magnified oblique multislice (ZOOM) EPI. In Proceedings of the 8th ISMRM Annual Meeting, Denver, CO, USA. 2000:p160.
- Wilm BJ, Svensson J, Henning A, Pruessmann KP, Boesiger P, Kollias SS. Reduced field-of-view MRI using outer volume suppression for spinal cord diffusion imaging. Magn Reson Med. 2007;57:625-630.
- Saritas EU, Cunningham CH, Lee JH, Han ET, Nishimura DG. DWI of the spinal cord with reduced FOV single-shot EPI. Magn Reson Med. 2008;60:468-473.
- Tian Y, Wang J, Li M, et al. Comparison of field-of-view optimized and constrained undistorted single-shot diffusion-weighted imaging and conventional diffusion-weighted imaging of optic nerve and chiasma at 3T. Diagnostic Neuroradiol. 2018; doi:10.1007/s00234-018-2058-5.
- Seeger A, Schulze M, Schuettauf, et al. Advanced diffusion-weighted imaging in patients with optic neuritis deficit – value of reduced field of view DWI and readout-segmented DWI. Neuroradiol J. 2018;0:1-7.
- Wang M, Liu H, Wei X, et al. Application of reduced-FOV diffusion-weighted imaging in evaluation of normal pituitary glands and pituitary macroadenomas. AJNR Am J Neuroradiol. 2018;39:1499-1504.
- Khant ZA, Minako A, Kadota Y, et al. Evaluation of pituitary structures and lesions with turbo spin-echo diffusion weighted imaging. J Neuroradiol Sci. 2019;405:116390.
- Deng J, Omary RA, Larson AC. Multishot diffusion-weighted SPLICE PROPELLER MRI of the abdomen. Magn Reson Med. 2008;59:947-953.
- Zhao X, Li Z, Gaddipati A. PROPELLER DUO: Applied to diffusion-weighted imaging. In Proceedings of the 17th ISMRM Annual Meeting, Honolulu, Hawaii, USA. 2009:p3518.
- Pipe JG, Farthing VG, Forbes KP. Multishot diffusion-weighted FSE using PROPELLER MRI. Magn Reson Med. 2002;47:42-52.
- Schick F. SPLICE: Sub-second diffusion-sensitive MR imaging using a modified fast spin-echo acquisition mode. Magn Reson Med. 1997;38:638-644.
Figures
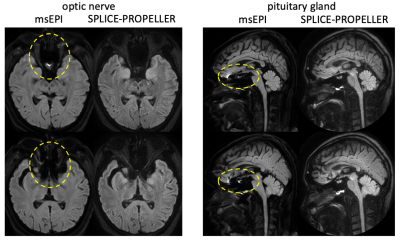
Fig. 1. Immunity of SPLICE-PROPELLER to geometric distortion artifacts. The distortion artifacts in msEPI (yellow ellipses) that often render the images of the optic nerve and pituitary gland non-diagnostic, are not present in SPLICE-PROPELLER.
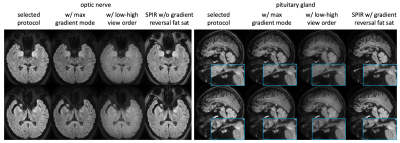
Fig. 2. Comparison of protocols. In each panel, the selected protocol is compared to 3 other options, one at a time. In the final protocol for optic nerve, selected options include enhanced gradient mode for high SNR (vs max mode), linear view order for sharp and uniform image (vs low-high order. Note that the images with low-high order are scaled by 50% to achieve similar window/level), SPIR with gradient reversal for better suppression of fat signals around the nerves. For the pituitary gland, gradient reversal is not used as it may lose some fine structures (such as the pituitary stalk).
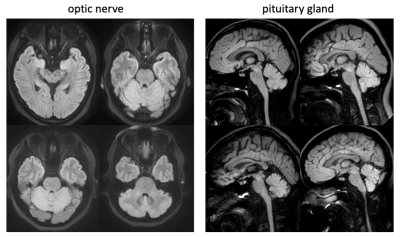
Fig. 3. Volunteer images acquired using the proposed SPLICE-PROPELLER protocols demonstrate good image quality without distortion artifacts.
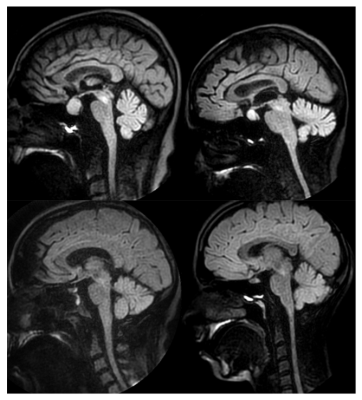
Fig. 4. Patient results of the pituitary gland with the proposed SPLICE-PROPELLER protocol. Clear delineation of the pituitary gland with consistent quality is achieved in the clinical settings.