3934
The efficacy 2D turbo gradient- and spin-echo diffusion-weighted imaging for cerebellopontine angle tumors1Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 2Hubei Province Key Laboratory of Molecular Imaging, Wuhan 430022, China, Wuhan, China, 3MR Collaboration, Siemens Healthcare Ltd., Guangzhou, China., Guangzhou, China, 4Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China., Shenzhen, China
Synopsis
Geometric distortions and ghosting artifacts are found at bone-air interfaces using conventional diffusion-weighted imaging (DWI), which is a challenge for imaging cerebellopontine angle (CPA) tumors. Our study validated that geometric distortions and ghosting artifacts were not present on 2D turbo gradient- and spin-echo-BLADE-DWI scans, making this technique useful for visualizing CPA anatomic structures and diagnosing CPA tumors.
Background and Purpose
We aimed to compare the clinical utility of the prototypic 2D turbo gradient- and spin-echo-BLADE-diffusion weighted imaging (TGSE-BLADE-DWI) with that of readout-segmented echo-planar DWI (RESOLVE-DWI) and single-shot echo-planar DWI (SS-EPI-DWI) to visualize cerebellopontine angle (CPA) anatomic structures and identify CPA tumors.Materials and methods
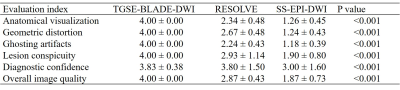
This study was approved by the local medical ethics committee, and written informed consent was obtained from each enrolled participant. Eight volunteers and 30 patients diagnosed with CPA tumors were enrolled in the study. We performed TGSE-BLADE-DWI, RESOLVE-DWI and SS-EPI-DWI using comparable voxel size for CPA on a 3 Tesla magnetic resonance (MR) scanner system (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) with a 20-channel head & neck coil. The detailed parameters for TGSE-BLADE-DWI, RESOLVE-DWI, and SS-EPI-DWI are listed in Table 1.Subjective evaluations: Table 2 lists the 4- or 5-points grading scales used for anatomic visualization, geometric distortion, the presence of ghosting artifacts, lesion conspicuity, diagnostic confidence, and overall image quality.
Objective evaluations: Lesion maximum diameters were recorded. Quantitative parameters, including signal-to-noise ratios (SNRs), contrast-to-noise ratios (CNRs), and apparent diffusion coefficients (ADCs) of the CPA tumors were compared among the three DWI methods. SNRs and CNRs were calculated using the following formulas:
SNR=SIlesion/SDbackground
CNR=(SIlesion-SIbrainstem)/√[(SDlesion)2+(SDbrainstem)2 ]
SIlesion and SIbrainstem represent the mean signal intensities of lesions and brainstems, respectively. SDbackground and SDbrainstem represent air and brainstem standard deviations, respectively. Lesions <5 mm were excluded from the quantitative assessments because it was difficult to place the regions of interest (ROIs) within those lesions. All the measurements were performed avoiding adjacent air, bone, and susceptibility artifact signals.
Results
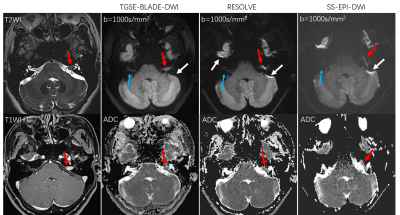
Thirty patients with 44 lesions were identified from the MR images, including 21 patients with 23 acoustic neuromas, six patients with 18 cholesteatomas, two patients with meningiomas, one patient with one epidermoid cyst. Forty-one lesions in 30 patients were observed with TGSE-BLADE-DWI. Of those, only three small cholesteatomas, with diameters <1 mm, were missed. In contrast, 11 lesions in six patients scanned with RESOLVE-DWI were missed due to geometric distortions and ghosting artifacts. Eighteen lesions were not identified in ten patients using SS-EPI-DWI.Subjective evaluations: Significant differences were found in all the quantitative parameters among the three DWI methods (all p <0.001, Table 3). TGSE-BLADE-DWI demonstrated better performance than RESOLVE-DWI (all p <0.005), except when examining comparable diagnostic confidence (p=0.131). Finally, RESOLVE-DWI was significantly superior to SS-EPI-DWI for all imaging parameters (all p <0.001). Figure 1 shows a representative comparison of the three DWI methods in one patient with an acoustic neuroma. The lesion was clearly visualized without geometric distortions in the TGSE-BLADE-DWI scans; however, moderate and severe distortions were seen with the RESOLVE-DWI and SS-EPI-DWI methods, respectively. The ADC values of this lesion on TGSE-BLADE-DWI, RESOLVE and SS-EPI-DWI were 0.95 x 10-3 mm2/s, 1.01 x 10-3 mm2/s, 1.04 x 10-3 mm2/s, respectively.
Objective evaluations: CNRs and apparent diffusion coefficients (ADCs) were not significantly different among the three DWI methods (p=0.329, p=0.797, respectively, Table 4), but a significant difference in SNRs was detected (p=0.019). RESOLVE-DWI produced significantly higher SNRs than SS-EPI-DWI (p=0.017), whereas no significant differences were detected between TGSE-BLADE-DWI and RESOLVE-DWI (p=0.203), and between TGSE-BLADE-DWI and SS-EPI-DWI (p=0.959).
Conclusions
Compared with RESOLVE-DWI and SS-EPI-DWI, TGSE-BLADE-DWI minimized geometric distortions and ghosting artifacts and demonstrated an improved ability to depict and diagnose CPA tumors.Acknowledgements
Not applicable.References
1. Zhou K, Liu W, Cheng S (2018) Non-CMPG PROPELLER diffusion imaging: comparison of phase insensitive preparation with split acquisition. In: Proceedings of the Annual Meeting of the International Society for Magnetic Resonance in Medicine; 5320.
2. Xu X, Wang Y, Hu H, Su G, Liu H (2017) Readout-segmented echo-planar diffusion-weighted imaging in the assessment of orbital tumors: comparison with conventional single-shot echo-planar imaging in image quality and diagnostic performance. Acta Radiol 58:1457–1467.
3. Kammer NN, Coppenrath E, Treitl KM, et al (2016) Comparison of contrast-enhanced modified T1-weighted 3D TSE black-blood and 3D MP-RAGE sequences for the detection of cerebral metastases and brain tumours. Eur Radiol 26:1818–1825.
4. Sheng Y, Hong R, Sha Y, et al (2020) Performance of TGSE BLADE DWI compared with RESOLVE DWI in the diagnosis of cholesteatoma. BMC Med Imaging 20:40.
5. Yuan T, Sha Y, Zhang Z, et al (2020) TGSE diffusion-weighted pulse sequence in the evaluation of optic neuritis: A comprehensive comparison of image quality with RESOLVE. Proc. Intl. Soc. Mag. Reson. Med. 28
Figures
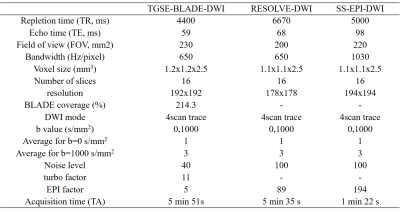
Table 1. Detailed parameters of the three diffusion-weighted imaging methods.
(Abbreviations: TGSE-BLADE-DWI, 2D turbo gradient- and spin-echo-BLADE-diffusion weighted imaging; RESOLVE-DWI, readout-segmented echo-planar DWI; and SS-EPI-DWI, single-shot echo-planar DWI )