3931
Childhood Brain Tumour Classification Through Proton Magnetic Resonance Spectroscopy and Diffusion Weighted Imaging1Institute of Cancer and Genomic Sciences, University of Birmingham, Birmingham, United Kingdom, 2Department of Oncology, Birmingham Children's Hospital, Birmingham, United Kingdom
Synopsis
Multi-modal functional imaging is expected to improve the classification of childhood brain tumours. Forty-three patients with a confirmed childhood brain tumour were enrolled in this 1.5T multi-modal functional imaging study. Short-echo proton magnetic resonance spectroscopy (1H-MRS) and diffusion weighted imaging (DWI) were acquired and analysed through multi-class receiver operating characteristics for feature selection and a wavelet-based data-driven framework for 1H-MRS noise suppression. The balanced classification accuracy across the three tumour types was improved to 95% through linear discriminant analysis by combining DWI and noise-suppressed 1H-MRS, showing improved from 84% through only DWI and 88% through only noise-suppressed 1H-MRS.
Introduction
Childhood brain tumours are amongst the most common solid cancer in children. Functional imaging plays a key role in clinical brain tumour management. Combined with machine learning, single-modality functional imaging achieves limited classification accuracy for imbalance childhood brain tumours1, while the classification performance based on multi-modal functional imaging was rarely reported. This study aims to improve childhood brain tumour classification by combining 1.5T short-echo proton magnetic resonance spectroscopy (1H-MRS) and diffusion weighted imaging (DWI) with state-of-the-art methods of quality improving and feature selection.Methods
Pre-surgical brain tumour imaging data were collected from thirty-three patients who were diagnosed as ependymoma (N=6), medulloblastoma (N=13) or pilocytic astrocytoma (N=14) during 2014-2019. The study was approved by the local research ethics committee (ethic number: 04/MRE04/41). Structural and functional imaging acquisition was performed on a 1.5T Philips Ingenia scanner at Birmingham Children’s Hospital during 2004-2019 by using the point-resolved spectroscopy sequence for 1H-MRS (B0 1.5T, TE 30‐35ms, TR 1500‐2000ms, voxel size 20-80mm3) and the echo planar imaging sequence for DWI (B0 1.5T, TE 89ms, TR 6100ms, acquisition time 1-3 mins, b-values 0 and 1000). Acquired 1H-MRS was processed by using a wavelet-based data-driven framework for noise suppression2-3 and TARQUIN for quantification. Regions of interests for brain tumours on DWI were drawn on b0 images with cysts excluded by experienced radiologists, reviewed by experienced oncologists, and reconstructed based on the vertices. Extracted features including metabolite profiles and apparent diffusion coefficients (ADC) were filtered to remove highly correlated features and ranked based on the area under the curve (AUC) through multi-class receiver operating characteristics (ROC) across the three brain tumour types. Machine learning experiments were conducted by using features no more than the minority group with linear discriminant analysis and support vector machine. The classification accuracy was determined by using leave-one-out and six-fold cross validation. An unpaired non-parametric Wilcoxon test was used to evaluate the significant difference for classification accuracy.Results
A series of ADC features and metabolite concentrations showed good diagnostic ability across the three tumour types. On the one hand, ADC features showing the highest diagnostic ability include ADC minimum (AUC=.98), 5th percentile ADC (AUC=.97), 20th percentile ADC (AUC=.97) and so on. On the other hand, myo-Inositol (AUC=.89), total lipids and macromolecules at 0.9ppm (TLM0.9, AUC=.85) and the combination of glutamate and glutamine (Glx, AUC=.84) were ranked as the metabolites with the best diagnostic ability before noise suppression, while myo-Inositol (AUC=.95), Glx (AUC=.86) and total creatine (tCr, AUC=.82) were ranked top after noise suppression. After combining the two modalities and excluding the highly correlated features, the top four features for distinguishing between tumour types were suggested to be minimum ADC, myo-Inositol, TLM0.9, and Glx before noise suppression, and minimum ADC, myo-Inositol, Glx, and tCr after noise suppression (Figure 3).Multi-modal imaging showed significantly better classification accuracy than single-modal imaging (P<.05, Figure 4-5). Taking the results of support vector machine as an example, the balanced classification accuracy determined through leave-one-out cross validation showed as 94% by combining DWI and 1H-MRS, in comparison to 83% using DWI and 86% using only noise-suppressed 1H-MRS.
Discussion
Progress has been made for 1H-MRS based childhood brain tumour classification in feature selection and quality improving. Based on those findings, this study optimised the methods of data processing and analysis for multi-modal imaging. Principal component analysis was used to perform dimensionality reduction for metabolite profiles in classic methods, while it could lead to the domination of one modality when combining the two modalities. On the country, multi-class ROC evaluates each feature individually and provides a generalised criteria to select features across the two modalities. Noise suppression is able to improve the signal-to-noise ratio of the 1H-MRS spectrum and presumably the accuracy of metabolite concentrations4. Therefore, the multi-modal functional imaging based classification performance can get improved through combining the two methods in machine learning.Ependymomas remain challenging to identify even with multi-modal imaging, due to its diversity and naturally biological structure. DWI is expected to initially classify ependymomas as either a medulloblastomas or a pilocytic astrocytomas, after which 1H-MRS is expected to refine the classification through key metabolite concentrations. Taking the advantages of the two modalities, ependymomas showed better classified through the key ADC metrics and metabolite concentrations and achieved improved balanced classification accuracy across the three tumour types.
Future work includes introducing wavelet-based oversampling to further improve the classification performance and implementing the current progress into the decision support system for clinical childhood brain tumour diagnosis.
Conclusion
The combination of diffusion weighted imaging and noise-suppressed proton magnetic resonance spectroscopy provides optimal classification performance for childhood brain tumours than single-modality.Acknowledgements
We would like to acknowledge funding from the Cancer Research UK and EPSRC Cancer Imaging Programme at the Children’s Cancer and Leukaemia Group (CCLG) in association with the MRC and Department of Health (England) (C7809/A10342), the Cancer Research UK and NIHR Experimental Cancer Medicine Centre Paediatric Network (C8232/A25261), Health Data Research UK (HDR UK) and the Children’s Research Fund.References
- Zarinabad N, Wilson M, Gill SK, et al. Multiclass imbalance learning: Improving classification of pediatric brain tumors from magnetic resonance spectroscopy. Magnetic Resonance in Medicine. 2017;77(6):2114-2124.
- Zhao D, Grist JT, Sun Y, et al. Impact of wavelets and apodisation in magnetic resonance spectroscopy quality for paediatric brain tumours. Proceedings of the Annual Meeting of ISMRM, Montréal, Canada 27(4243).
- Zhao D, Grist JT, Sun Y, et al. Optimised paediatric brain tumor diagnosis by using in vivo magnetic resonance spectroscopy and machine learning. Proceedings of the Annual Meeting of ISMRM, Sydney, Australia 28(1394).
- Zhao D, Grist JT, Sun Y, et al. Improved classification of paediatric brain tumours through whole spectra from in vivo magnetic resonance spectroscopy. Proceedings of the Annual Meeting of ISMRM, Montréal, Canada 27(3083).
Figures
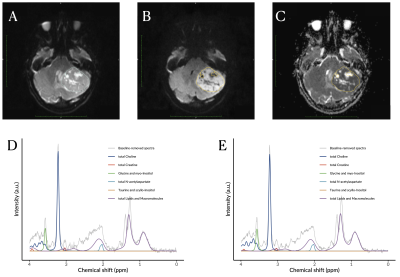
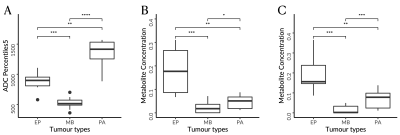
Images showing the significance of key features in discriminating the three childhood brain tumours, given by the fifth percentile apparent diffusion coefficient (A) and myo-Inositol from noisy (B) and noise-suppressed (C) proton magnetic resonance spectroscopy.
Abbreviations: ADC, apparent diffusion coefficients; EP, ependymomas; MB, medulloblastomas; PA, pilocytic astrocytomas.
Levels of significance: *, P<.05; **, P<.01; ***, P<.001; ****, P<.0001.
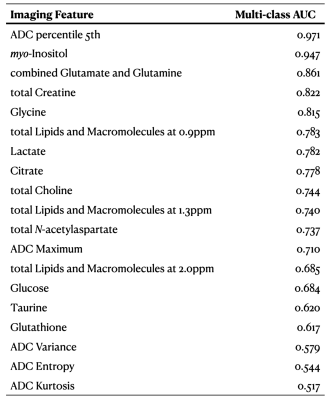
List of significant multi-modal imaging features for classifying childhood brain tumours, showing the diagnostic ability across the three tumour types by multi-class area under the curve.
Abbreviations: ADC, apparent diffusion coefficients; AUC, area under the curve.
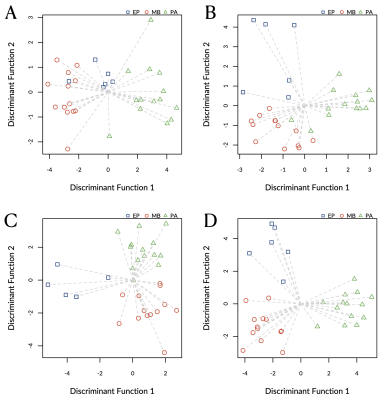
Classification map derived through re-substitution and linear discriminant analysis with (A) only diffusion weighted imaging (DWI), (B) only noisy proton magnetic resonance spectroscopy (1H-MRS), (C) only noise-suppressed 1H-MRS, or (D) combined DWI and noise-suppressed 1H-MRS.
Abbreviations: EP, ependymomas; MB, medulloblastomas; PA, pilocytic astrocytomas.
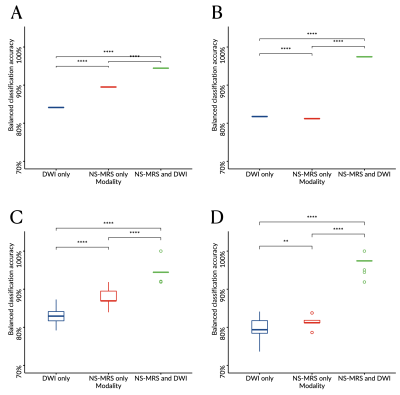
Boxplots comparing the balanced classification accuracy estimated through leave-one-out (A-B) or six-fold (C-D) cross validation and determined through linear discriminant analysis (A, C) or support vector machine (B, D) from diffusion weighted imaging (DWI) only, noise-suppressed proton magnetic resonance spectroscopy (NS-MRS) only, or combining NS-MRS and DWI.
Levels of significance: *, P<.05; **, P<.01; ***, P<.001; ****, P<.0001.