3929
Differentiation of benign and malignant neck tumors by APT1the First Affiliated Hospital of Dalian Medical University, Dalian, China
Synopsis
Neck malignant tumors is the seventh most common cancer in the world and the ninth most common cause of cancer death. Meanwhile, challenges remain in the differential diagnosis of benign and malignant tumors in the neck. In this study, we explored the value of APTw imaging in the differential diagnosis between malignant and benign neck tumors. Results showed significantly higher APTw values in the malignant tumors than in benign tumors (AUC=0.882, P=0.006), suggesting APT a promising non-invasive method in the differentiation of the neck malignant and benign tumors.
Introduction
Neck cancer is the seventh most common cancer in the world and the ninth most common cause of cancer death. The biological nature of neck tumors determines the treatment strategy and prognosis, and many MR techniques have been used in neck tumors evaluations, such as diffusion weighted imaging (DWI) and dynamic contrast-enhanced (DCE) MRI. However, DWI quality is often degraded by magnetic susceptibility artifacts in the neck, leading to geometric distortion and low spatial resolution, and safety issues could be associated with the use of contrast media in DCE-MRI. Therefore, the differential diagnosis of benign and malignant tumors in the neck remains challenging for MRI. On the other hand, amide proton transfer-weighted (APTw) MRI provides a scope of endogenous intracellular free proteins and polypeptides, the potential of which has been shown in tumor detection, diagnosis and treatment. In this study, we measured the APT values of neck tumors to differentiate the malignant tumors from benign lesions.Summary of Main Findings
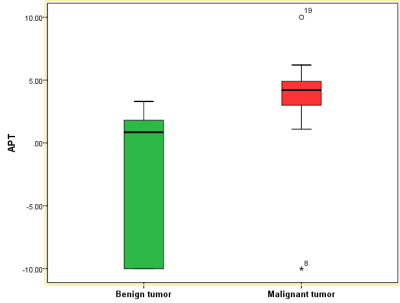
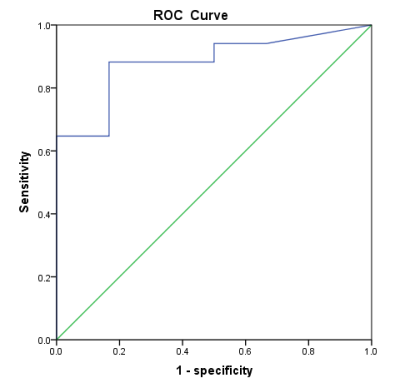
Neck tumor APTw values of the malignant group were significantly higher than those of the benign group. The ROC curve to differentiate between the malignant and benign tumors had an AUC value of 0.882, with a cut-off at 1.95%, a sensitivity of 0.882 and a specificity of 0.833.Methods
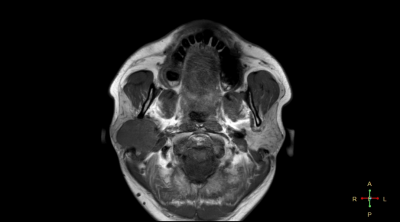
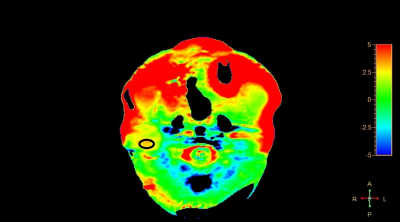
This retrospective study was approved by the institutional IRB and the written informed consent was waived. Twenty-three patients with neck tumors (7 benign and 16 malignant, surgically confirmed) and were included, who underwent pre-operative MRI at 3.0T (Ingenia CX, Philips Healthcare, the Netherlands) including a 3D APTw sequence (TR/TE=3000/7.9 ms, FOV=230x221x62 mm3, resolution=2.5x2.5x2.5 mm3, acquisition matrix=120×140×40, scan duration = 481 s). For each patient, two radiologists (Wang Lijun and Chen Lihua, with 20 and 5 years radiology experiences respectively) independently measured the tumor APTw values (MTRasym) by placing the ROIs (25-64 mm2) on the slice that shows the largest area of the lesion while avoiding blood vessels as much as possible (Figure 1), with reference to the T2w images. The interobserver reliability on qualitative evaluation was assessed via Cohen’s kappa test (excellent agreement if k > 0.9; good agreement if k > 0.6). In case of good agreement, the mean values of the measurements from the two observers were used for the subsequent analysis. One sample Kolmogorov-Smirnov test was performed to test the normality of the mean APTw values. The differences of the mean APTw values between the malignant and benign tumors were compared by using the Mann-Whitney U test. Receiver operating characteristic (ROC) analysis was used to determine the value of mean APTw value for the differentiation of malignant tumors from benign tumors. The area under curve (AUC) of mean APTw values were compared by Z test.Results
Measurement consistency between the two observers was good (Kappa=0.324, p<0.001). The mean APTw values did not conform to normal distribution using K-S test (P<0.05). APTw values of the malignant group were significantly higher than those of the benign group (p < 0.05) (Figure 1). For the differentiation between malignant and benign groups, the ROC curve analysis yielded an AUC value of 0.882 (P=0.006) (Figure 2, cut-off at 1.95%), with a sensitivity of 0.882 and a specificity of 0.833.Figure 1. The measurement of the mean APTw Values in neck tumors.
Figure 2. APTw values of the cervical malignant tumor group were significantly higher than those of the benign tumor group.
Figure 3. The ROC curve of the APTw value to differentiate between the malignant and benign neck tumors.
Discussion and conclusion
The APTw values of neck malignant tumors were significantly higher than that of benign tumors, which was in line with our expectation. Malignant tumor cells proliferate more rapidly and require increased protein synthesis, providing the basis of the increased APTw values. Similar results are obtained in the comparison of benign and malignant tumors in other body parts . Since MRI in the neck usually suffers from the B0 field inhomogeneity, a TSE acquisition was exploited in our study to retain the image quality and measurement robustness. Our results suggested the potential of APTw imaging in the differential diagnosis between malignant and benign neck tumors with relatively high sensitivity and specificity.Acknowledgements
No acknowledgement found.References
[1] Choi YS, Ahn SS, Lee S-K, et al. Amide proton transfer imaging to discriminate between low- and high- grade gliomas: added value to apparent diffusion coefficient and relative cerebral blood volnme [J]. European Radiology. 2017,27(8):3183-3189.
[2]. Sakata A, Fushimi Y, Okada T, et al. Diagnostic performance between contrast enhancement, proton MR spectroscopy, and amide proton transfer imaging in patients with brain tumors: CE, MRS, and APT imaging of brain tumors [J]. Journal of Magnetic Resonance Imaging. 2017,46(3):732-739.
[3]. Sun PZ, Zhou J, Sun W, Huang J, van Zijl PC. Detection of the ischemic penumbra using ph-weighted MRI[J]. Journal of Cerebral Blood Flow & Metabolism. 2017,27(6):1129-1136.
[4]. Sun PZ, Zhou J, van Zijl P. Simplified quantitative description of amide proton transfer (APT) imaging during acute ischemia [J]. Magnetic Resonance in Medicine. 2007,57(2):405-410.
[5]. Joshi P, Benussi L, Furlan R, Ghidoni R, Verderio C. Extracellular vesicles in Alzheimer’s Disease: friends or foes? Focus on Aβ-vesicle interaction. International Journal of Molecular Sciences[J].
[6]. Haris M, Singh A, Cai K, et al. A technique for in vivo mapping of myocardial creatine kinase metabolism. Nature Medicine. 2014,20(2):209-14.
[7]Kogan F, Haris M, Debrosse C, et al. In vivo chemical exchange saturation transfer imaging of creatine (CrCEST) in skeletal muscle at 3T: Muscle CrCEST at 3T [J]. Joiamal of Magnetic Resonance Imaging. 2014,40(3):596-602.