3928
Negative-contrast neurography of the extracranial facial nerve and branches based on variable flip angle turbo spin echo imaging1Centre for Medical Imaging, University College London, London, United Kingdom, 2Department of Imaging, University College London Hospital, London, United Kingdom, 3Medical Physics, University College London Hospital, London, United Kingdom, 4Head and Neck Academic Centre, University College London, London, United Kingdom
Synopsis
Injury to the facial nerve is a major risk during parotid surgery; pre-operative identification of its course is key to minimising complications. Unfortunately, conventional MRI sequences offer insufficient resolution whilst conventional ‘positive-contrast’ neurographic methods suffer from artifacts and inconsistent quality. We propose an approach to imaging the extracranial facial nerve at high resolution using variable flip angle turbo spin echo imaging. This method depicts the nerve as a low-signal structure (‘black nerve’) against the high-signal parotid parenchyma (‘white parotid’). We show that this ‘negative-contrast’ method outperforms a widely-used positive-contrast neurographic sequence and might be further improved using gadolinium-based contrast agent.
Introduction
Injury to the facial nerve is a major risk during parotid surgery, and up to 35% of patients experience postoperative weakness following parotidectomy [1]. As a result, efforts have been made to develop MRI techniques for visualising the facial nerve course, to help plan surgery and thus to minimise the risk of facial nerve injury. Unfortunately, conventional MRI sequences offer insufficient spatial resolution for facial nerve imaging. Dedicated neurographic methods such as the double echo steady state (DESS) sequence have shown promise for facial nerve imaging and typically show the nerve as a high-signal structure against the low-signal parotid gland [2–5]. However, these sequences can perform poorly where the parotid is less fatty since the intrinsic contrast between nerve and gland is reduced and are highly susceptible to inadequate fat suppression/water excitation due to B0 inhomogeneity.We propose a ‘negative-contrast’ approach to imaging the extracranial facial nerve based on variable flip angle turbo spin echo (VFA-TSE) imaging. We compared this method to a conventional ‘positive-contrast’ technique and evaluated the effect of parotid-specific optimisation and of gadolinium-based contrast on the contrast-to-noise ratio of the nerve against the parotid parenchyma.
Methods
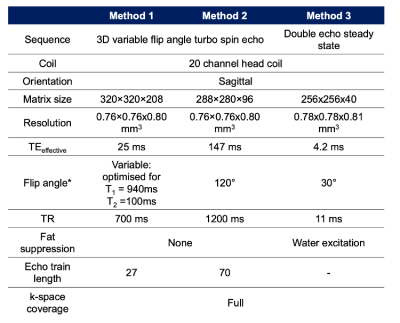
All imaging was performed on a 3.0T Siemens Vida system with a standard 20-channel head coil. We first compared two implementations of the VFA-TSE (Siemens SPACE) sequence (Figure 1, Methods 1 and 2), optimised to provide T1-weighting and T2-weighting respectively, against a double echo steady state (DESS) sequence with water excitation (Figure 1, Method 3) in six volunteers. The best-performing acquisition in terms of contrast-to-noise (CNR) was then further optimised by testing the effect of T1 optimisation on CNR. To evaluate the effect of contrast on CNR, imaging was performed in ten patients with parotid tumours due to undergo surgery, both before and after gadolinium-based contrast agent (GBCA); hand injection of 10ml of gadoteridol 279.3 mg/ml). In these patients, both the normal side and the side with the tumour were imaged.The resulting images of the extracranial facial nerve were manually segmented by two consultant head and neck radiologists, placing regions-of-interest placed on the main trunk and divisions of the facial nerve in addition to the parotid duct and retromandibular vein. Specifically, ROIs were placed on (1) the main trunk, (2) upper division and branches (3) lower divisions and branches and (4) the buccal branch. The parotid parenchyma was segmented by a single additional observer in order to provide a constant signal intensity value (denoted ) for contrast-to-noise ratio (CNR) measurement for each of the two primary readers. Images were compared in terms of contrast-to-noise ratio of the nerve against the parotid gland and reader confidence. CNR for the nerve was defined as
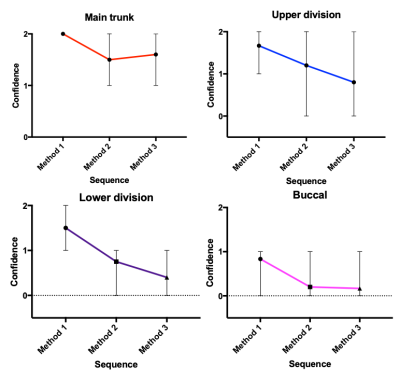
$$CNR =\frac{S_{nerve}- S_{parotid}}{\sqrt{({SD_{nerve}}^{2}+ {SD_{parotid}}^{2})/2}}$$ where $$$S_{nerve}$$$ and $$$S_{parotid}$$$ are the signals from the nerve and parotid masks respectively and $$$SD_{nerve}$$$ and $$$SD_{parotid}$$$ are the corresponding standard deviation values. The magnitude of the CNR was compared between sequences using a mixed-effects regression analysis over all nerve segments, accounting for missing values where segments could not be seen and so were not segmented. Reader confidence was recorded in terms of nerve visibility (0 – not seen, 1 – partially seen, 2 – fully seen) and compared between sequences.
Results and Discussion
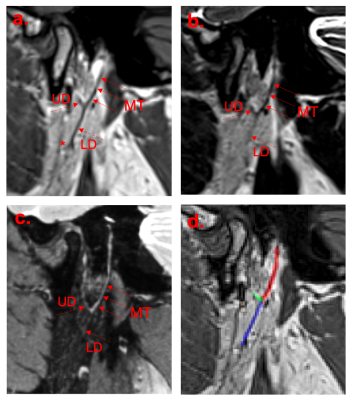
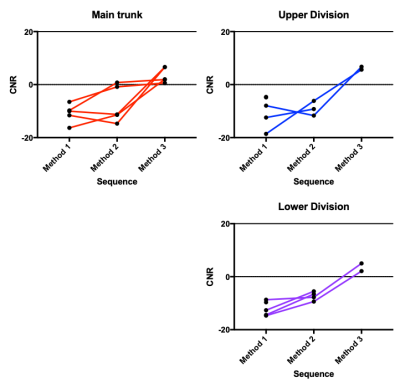
Example images from the three initial candidate methods and corresponding manual segmentation are shown in Figure 2. CNR values are compared by sequence in Figure 3, and confidence scores are compared in Figure 4. CNR magnitude was significantly higher for Method 1 (i.e. T1-weighted VFA-TSE) than Methods 2 or 3 (P=0.019). The greater CNR for Method 1 may reflect the fact that the parotid parenchyma has significant fat content, which is best captured by a sequence with T1 weighting, whereas the nerve has little or no fat (excluding myelin).For the optimisation experiment, there was no significant change in CNR depending on the T1 value used for sequence optimisation i.e. parotid-specific optimisation produced no benefit compared to standard settings.
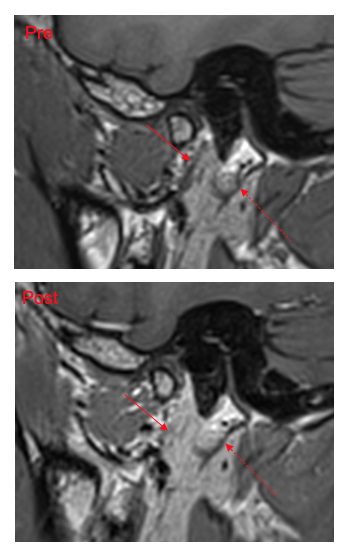
Pre- and post-GBCA of a patient (normal side), demonstrating enhancement of the parotid parenchyma and small vessels, are shown in Figure 5. There was a small improvement in contrast-to-noise ratio (P=0.1) and in confidence scores for the post-GBCA imaging compared to the pre-GBCA imaging. This probably reflects the fact that the parotid parenchyma and small vessels enhance with GBCA whereas the facial nerve itself does not. The use of GBCA therefore represents a possible means to further increase the negative-contrast property of the methodology and to remove potential distractors in nerve identification. Further work aiming to maximise the enhancement of intraparotid vessels and thus increase the homogeneity of non-nerve parotid structures would be of value.
Conclusions
Imaging of the extracranial facial nerve using VFA-TSE imaging offers superior performance to an equivalent positive-contrast neurographic technique. The proposed method generates negative contrast between the nerve and parotid and depicts the nerve as a low-signal structure (‘black nerve’) against the high-signal parotid parenchyma (‘white parotid’). Negative contrast between nerve and parotid might be further increased using GBCA. This approach could help to plan surgery in patients with parotid tumours.Acknowledgements
TJPB is supported by an NIHR Clinical Lectureship. MHC was supported by the UCLH Biomedical Research Centre (BRC). This work was undertaken at UCLH/UCL, which receives funding from the UK Department of Health’s the National Institute for Health Research (NIHR) BRC funding scheme. The views expressed in this publication are those of the authors and not necessarily those of the UK Department of Health.References
1. Sood AJ, Houlton JJ, Nguyen SA, Gillespie MB. Facial nerve monitoring during parotidectomy: A systematic review and meta-analysis. In: Otolaryngology - Head and Neck Surgery (United States). 2015. p. 631–7.
2. Qin Y, Zhang J, Li P, Wang Y. 3D double-echo steady-state with water excitation MR imaging of the intraparotid facial nerve at 1.5T: A pilot study. Am J Neuroradiol. 2011;32(7):1167–72.
3. Fujii H, Fujita A, Kanazawa H, Sung E, Sakai O, Sugimoto H. Localization of parotid gland tumors in relation to the intraparotid facial nerve on 3D double-echo steady-state with water excitation sequence. Am J Neuroradiol. 2019;40(6):1037–42.
4. Guenette JP, Ben-Shlomo N, Jayender J, Seethamraju RT, Kimbrell V, Tran NA, et al. MR imaging of the extracranial facial nerve with the CISS sequence. Am J Neuroradiol. 2019;40(11):1954–9.
5. Chu J, Zhou Z, Hong G, Guan J, Li S, Rao L, et al. High-resolution MRI of the intraparotid facial nerve based on a microsurface coil and a 3d reversed fast imaging with steady-state precession DWI sequence at 3T. Am J Neuroradiol. 2013;34(8):1643–8.
Figures