3926
Multi-parametric MRI in differentiation between brain tumor and radiation necrosis1Institute of Imaging Science, Vanderbilt University Medical Center, Nashville, TN, United States, 2Radiation Oncology, Vanderbilt University Medical Center, Nashville, TN, United States, 3Keller Center for Imaging Innovation, Barrow Neurological Institute, Phoenix, AZ, United States
Synopsis
It remains a challenge to differentiate between recurrent tumors from radiation induced necrosis in the brain. One promising approach is to combine complementary information on different scales obtained using multi-parametric MRI to comprehensively characterize tissue histopathological properties. In this preliminary study, we performed six multi-parametric MRI acquisitions including APT (probing mobile proteins), NOE (mobile macromolecules), qMT (macromolecules), ADC (cell density), SSIFT (cell size), and DSC (perfusion) to differentiate 9L gliosarcoma from radiation necrosis in animal models. The results suggest APT and SSIFT provide the best discrimination of different tissue types, but a larger sample size is required for further validation.
Introduction
It remains a clinical challenge to differentiate recurrent tumors and radiation induced necrosis (RN) in the brain. In addition to extensive efforts to develop new imaging methods to tackle this issue, another promising strategy is to combine complementary information on different scales obtained using multi-parametric MRI to comprehensively characterize tissue histopathological properties. In this work, six different MRI protocols were implemented and compared to differentiate brain tumors from RN. These methods were:- APT (amide proton transfer) probing mobile proteins,
- NOE (nuclear Overhauser effect) probing mobile macromolecules,
- qMT (quantitative magnetization transfer) probing macromolecules,
- ADC (apparent diffusion coefficient) probing cell density,
- SSIFT (selective size imaging using filters via diffusion times) probing cell size differences, and
- DSC (dynamic susceptibility contrast) probing vascular perfusion.
Methods
All acquisitions were performed on a 4.7T Varian/Agilent preclinical MRI scanner. Two groups of Fisher 344 rats were scanned with multi-parametric MRI: one group (n=5) developed radiation necrosis in the brain following irradiation using a clinical LINAC and the other group (n=4) developed 9L gliosarcoma brain tumors. All studies were approved by the local IACUC. The specific acquisition protocols and data analyses for each method are summarized below.- APT and NOE used a 5-second saturation pulse with 1 μT and 50 frequency offsets evenly distributed in the range of -50 to 50 ppm. A six-pool (amide, amine, NOE(-1.6), NOE(-3.5), MT, and water) model Lorentzian fitting was performed to achieve the APT peak at 3.5 ppm and the NOE peak at -3.5ppm.
- qMT used the selective inversion recovery method which employs a 1-ms hard pulse for inversion followed by a single-shot EPI readout. An optimized 7-pairs of ti (inversion time) and td (delay time) were used and a bi-exponential SIR model was fit to data to estimate PSR (pool size ratio of immobilized protons over free water protons).
- ADC was obtained with conventional 32-direction diffusion tensor imaging with b = 1000 mm2/ms.
- SSIFT was achieved using a diffusion time range from 10ms to 70 ms and b = 1000 mm2/ms. The incremental area under curve (iAUC) can be obtained which is sensitive to cell size differences.
- DSC was acquired using single-echo GRE with TR = 15.6 ms, TE = 5 ms, flip angle = 9º. A 30-minute time-course was generated with 1 sec resolution following a 0.2 mmol/kg Gd bolus injected via jugular catheter. Relative cerebral blood volume (rCBV) was fitted.
Results
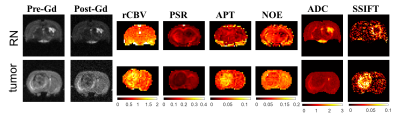
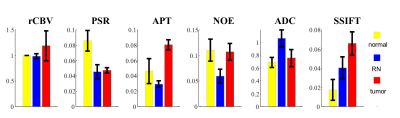
Figure 1 shows representative multi-parametric maps of two rat brains with an RN in the left hemisphere (top) and a 9L tumor in the right hemisphere (bottom). The tumor regions were manually drawn based on pre- and post-Gd images. The increases of rCBV, APT, and SSIFT in tumors are consistent with previous reports. Interestingly, rCBV does not provide sufficient contrast for RN while PSR provides the best contrast.Figure 2 summarizes all ROI-based MRI parameters in the differentiation of contralateral normal appearing brain tissue (n=9), RN (n=5), and 9L brain tumor (n=4). rCBV is slightly higher in tumors than in normal and RN (p=0.18) and is almost indistinguishable in normal and RN. PSR provides an excellent contrast to differentiate lesions from normal tissues, but it cannot distinguish tumors from RN. Both NOE and ADC shows high sensitivity to distinguish RN from other tissues (all p ≤ 0.03), although tumors show similar results as in normal tissues. APT and SSIFT provide the best discrimination to differentiate three different tissue types from each other (all p≤0.03).
Discussion and Conclusion
Our results suggest APT and SSIFT provide the best discrimination of normal appearing brain tissues, radiation necrosis, and tumors. There are several limitations to the current work. The sample size needs to increase for more reliable results. More brain tumor models are desirable to evaluate the robustness of mpMRI with significant pathological variations. The integration of different MRI metrics into a multi-parametric model may provide a more reliable differentiation of tissue types.Acknowledgements
This work was supported by the Vanderbilt Radiology/VUIIS Catalyst Award Program.References
- K. Mitsuya et al., Perfusion weighted magnetic resonance imaging to distinguish the recurrence of metastatic brain tumors from radiation necrosis after stereotactic radiosurgery. Journal of neuro-oncology 99, 81-88 (2010).
- J. Zhou et al., Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nature medicine 17, 130-134 (2011).
- W. R. Masch et al., Comparison of Diffusion Tensor Imaging and Magnetic Resonance Perfusion Imaging in Differentiating Recurrent Brain Neoplasm From Radiation Necrosis. Academic radiology 23, 569-576 (2016).
- H. Mehrabian, K. L. Desmond, H. Soliman, A. Sahgal, G. J. Stanisz, Differentiation between Radiation Necrosis and Tumor Progression Using Chemical Exchange Saturation Transfer. Clinical cancer research : an official journal of the American Association for Cancer Research 23, 3667-3675 (2017).
- J. Xu et al., in Proceedings of the 26th Annual Meeting of ISMRM. (Paris, France, 2017), pp. 953.
Figures