3924
Detection of Cystathionine, 2-Hydroxyglutarate and Citrate in Oligodendrogliomas at 7T using Long-TE Semi-LASER1School of Health Sciences, Purdue University, West Lafayette, IN, United States, 2Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN, United States, 3Wellcome Centre for Integrative Neuroimaging, Nuffield Dept of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 4University of Oxford, Oxford, United Kingdom
Synopsis
Given the rapid pace of discoveries in glioma genomics and metabolomics, it is likely that improvements in diagnostic imaging using UHF MRS can be made by systematically reanalyzing previously generated data in light of new knowledge. In this study, we tested this by reanalyzing existing UHF MRS spectra using a refined basis set that included the potential oncometabolite cystathionine
Introduction
Diffuse gliomas are the most common primary brain tumor. Biopsy represents the gold standard for diagnosis and classification and will remain part of standard of care (1). However, biopsies are invasive, static, and prone to sampling bias, and are therefore unsuitable for disease monitoring and capturing tumor heterogeneity. There is a clear need for non-invasive clinical biomarkers that are: (a) robustly diagnostic at the molecular level, (b) quantitative, (c) prognostic, (d) predictive, and (e) dynamic. Accumulating evidence suggests that MRS at ultra-high field (UHF) can meet these needs by providing robust molecular feature elucidation in vivo and offering a novel strategy for diagnostic imaging in glioma patients (2).Given the rapid pace of discoveries in glioma genomics and metabolomics, it is likely that improvements in diagnostic imaging using UHF MRS can be made by systematically reanalyzing previously generated data in light of new knowledge. It has been recently demonstrated that cystathionine can be detected in vivo via MRS and it is proposed to be a potential biomarker for 1p/19q co-deleted gliomas (3). If confirmed, this approach effectively would allow us to make a non-invasive diagnosis of oligodendroglioma (IDH-mutant and 1p/19q codeleted) versus astrocytoma (IDH-mutant but 1p/19q wildtype) (4). This is clinically relevant as oligodendrogliomas have a better prognosis than astrocytomas and benefit from supramaximal neurosurgical resection. In this study, we tested this by reanalyzing existing UHF MRS spectra using a refined basis set that included the potential oncometabolite cystathionine (2).
Methods
A total of 20 glioma patients were recruited (age= 37±11, 13 males) for a 7T MRI scan. Written and informed consent was obtained from all participants in the study, which was approved by the relevant institutional ethics committee. As assessed by immunohistochemical and DNA sequencing from surgical tissue biopsies, 15 patients (age = 41±10, 12 males) were identified as carrying isocitrate dehydrogenase 1 (IDH1) R132H mutation (4 of them with 1p/19q co-deletion) and 5 (age = 27±5, four females) with a rare IDH2 variant and 1p/19q co-deletion. A semi-localization by an adiabatic selective refocusing (semi-LASER) pulse sequence was performed within each voxel of interest (VOI) for spectra measurements (5). The spectroscopy acquisition parameters were as below: volume size = 20 x 20 x 20 mm3 = 8 mL, TE = 110 ms, TR = 5-6 s, number of transients NT = 128, spectral bandwidth = 6 kHz, and data points = 2048. LCModel analysis with a refined basis set that included cystathionine, 2-hydroxyglutarate (2-HG), and citrate was applied for metabolite quantification. Metabolite concentrations are reported relative to the internal reference of total choline (tCho).Results
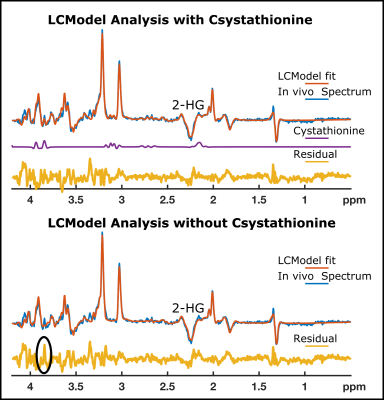
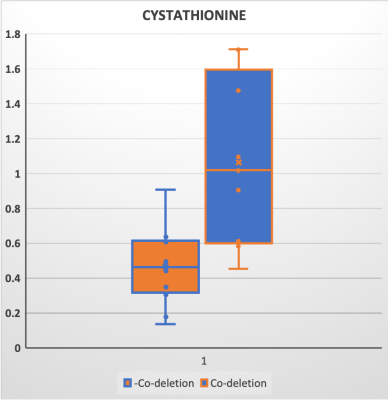
Due to the negative spectral pattern of 2-HG at a TE of 110 ms, and increased chemical shift dispersion at 7T, the cystathionine signal was clearly discernible in addition to the 2-HG signal in spectra of the tumor voxel in glioma patients with 1p/19q codeletion (Figure 1). The quantification of cystathionine using a refined LCModel basis set is illustrated in Figure 2. In line with a recent study (3), 1p/19q co-deleted gliomas have a significantly higher level of cystathionine/tCho (1.06±0.48 vs. 0.46±0.22, P<0.002).Conclusion and Discussion
The semi-LASER sequence optimized for 2-HG detection with a TE of 110 ms successfully demonstrated distinct cystathionine peaks in glioma patients with molecularly defined oligodendroglioma (IDH-mutant and 1p/19q codeleted) at 7T. While a prospective, better-powered study is needed to confirm our observations, we propose that our method has the potential to allow presurgical stratification of patients with IDH-mutant glioma into those with oligodendrogliomas and astrocytomas; which is of important prognostic significance.Acknowledgements
No acknowledgement found.References
1. Young RM, Jamshidi A, Davis G, Sherman JH. Current trends in the surgical management and treatment of adult glioblastoma. Annals of Translational Medicine 2015;3:9–9 doi: 10.3978/j.issn.2305-5839.2015.05.10.
2. Shen X, Voets NL, Larkin SJ, et al. A Noninvasive Comparison Study between Human Gliomas with IDH1 and IDH2 Mutations by MR Spectroscopy. Metabolites 2019;9 doi: 10.3390/metabo9020035.
3. Branzoli F, Deelchand DK, Sanson M, Lehéricy S, Marjańska M. In vivo 1H MRS detection of cystathionine in human brain tumors. Magnetic Resonance in Medicine 2019;82:1259–1265 doi: https://doi.org/10.1002/mrm.27810.
4. van den Bent MJ, Chang SM. Grade II and III Oligodendroglioma and Astrocytoma. Neurol Clin 2018;36:467–484 doi: 10.1016/j.ncl.2018.04.005.
5. Emir UE, Larkin SJ, de Pennington N, et al. Noninvasive quantification of 2-hydroxyglutarate in human gliomas with IDH1 and IDH2 mutations. Cancer research 2016;76:43–49.