3922
Applying 2HG MRS in glioma patients during routine clinical MRI examination1Department of Radiology, University Medical Center Utrecht, Utrecht, Netherlands, 2Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States
Synopsis
2HG is a valuable biomarker to detect mutational status of glioma patients. Therefore, it is of great value to be widely integrated into clinical practice. This study investigates the performance of along echo time (PRESS 97ms) 1H MRS protocol at 3Tesla to be used in routine clinical glioma imaging.
Introduction
Cancer associated IDH-mutation produces 2-hydroxyglutarate (2HG) in glial tumor cells1. Patients with IDH-mutated gliomas have improved overall survival rates compared to IDH-wildtype. Therefore, 2HG detection may aid the diagnosis and treatment planning2. 2HG can be detected by dedicated 1H-MRS (MR spectroscopy) sequences at 3T3–5. MRS, however, is not often applied in routine clinical protocols; hence successful data acquisition requires additional training of technicians which in return hampers adoption in the clinical routine. To get out of this vicious circle, we aimed to implement 2HG-MRS in the clinical routine and show the success rate and robustness of the protocol with minimal training. We included the 2HG protocol in clinical examinations at University Medical Center Utrecht. The measurements were performed by technicians, guided by instructions from experienced MR spectroscopists. Here, we present the results of a consecutive series of patients with glioma.Methods
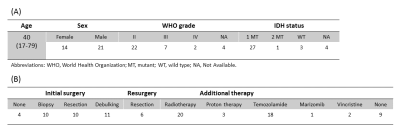
Patient populationWe performed 2HG MRS in 42 consecutive patients with proven or suspected glioma. Six patients were imaged twice (Table 1).
Data acquisition and analysis
Acquisitions were performed on a 3T scanner (Philips). A 2HG-optimized MRS protocol was implemented according to Choi et. al3 with the parameters:TE/TR=97ms/2s, spectral width=2500Hz, 2048 samples, 128 averages, scan time=5.5 min. The MRS voxel was positioned using T2w-FLAIR images. Technicians were instructed to a) avoid including liquor, skull and cystic part of the lesion in the MRS voxel; b) position the MRS voxel entirely within the tumor volume to reduce the partial volume effect; c) bound the shim box to the voxel and avoiding the air tissue interfaces inside the shim volume. Data were preprocessed and analyzed as described by Choi et al3, using LCModel6. The effect of relaxation rates was ignored. The unsuppressed water signal was used as a reference for absolute metabolite quantifications, assuming an in-vivo water concentration of 43.3 M. Signal to noise ratio (SNR) and linewidth were reported by LCModel. Partial volume of the tumor in the MRS voxel was estimated by a neuroradiologist as the percentage of the MRS voxel filled with the FLAIR abnormality. The majority of patients were imaged after resection and radiotherapy. This results in uncertainty regarding presence of actual tumor tissue in the voxel. Therefore, a likelihood of actual tumor being present within the voxel was classified as 0, 25, 50, 75 or 100 percent, blinded for the MRS results.
Assessment of robustness of 2HG detection
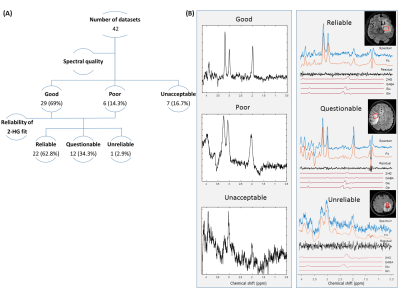
Three experienced MR spectroscopists assessed the spectra quality as good, poor (LW>14Hz) and unacceptable (SNR<3 compared to mean SNR of ~25, large water residual or baseline distortions). Also the reliability of the 2HG fit, regardless of the Cramér-Rao lower bound, was evaluated as ‘Reliable’, ‘Questionable’ or ‘Unreliable’.
Results
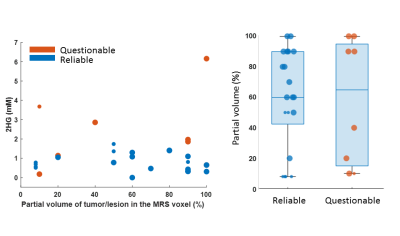
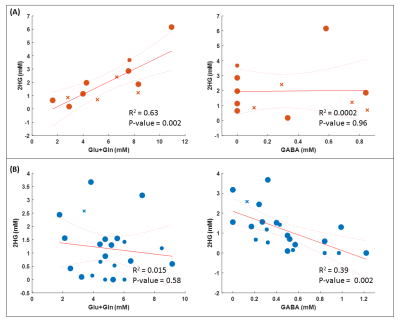
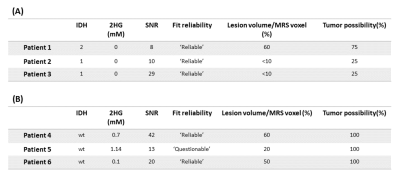
We excluded 8 datasets because of an unacceptable spectral quality and consequently an unreliable fit (Figure 1). The average linewidth and SNR after removal of unacceptable/unreliable spectra were 5.21±1.46 Hz and 25.31±12.8 respectively. MRS voxel size was 2.25-10.84 cm3. The minimum level of detected 2HG was 0.1 mM from a voxel of 3cm3 where 50% of the voxel filled with lesion with 100% chance of being tumor (IDH-wt). The highest level of 2HG was 6.16 mM from a voxel size of 7.5cm3 with 100% likelihood of the entire voxel contained tumor tissue. The amount of 2HG quantified in the ‘Reliable’ group is not biased with the percentage of the voxel occupied by the lesion (Figure 2). We observed that the detected 2HG level correlated with high Glx (Glutamate+Glutamine) signal in the ‘Questionable’ 2HG fits, which was in contrast to the ‘Reliable’ group (Figure 3). For 3 of the IDH-mutant patients, 2HG was not detected (Table 2A). For patient 1, this can be related to a very low SNR (voxel size 2.51cm3). The other two cases have a partial volume of <10% with a tumor possibility of 25%. In all IDH-wildtype data, 2HG was fitted (Table 2B). Regarding Patient 5, 2HG might be overestimated by Glx signal (according to the correlation in Figure 3B).Discussion
2HG detection can be of interest in at least two cases; detecting residual or recurrent IDH mutated tumors, or identifying low grade gliomas, most of which have IDH mutations, in epilepsy patients. We included both cases in this study. In our view it is possible to implement robust 2HG-MRS in clinical routine. We excluded 8 out of 42 spectra, however 7 of them are from the first 20 cases when some of the technicians performed 2HG-MRS for the first time. We observed that the 2HG level correlated with high Glx signal in ‘Questionable’ fits. This happened particularly when there was a high level of Glutamate in the voxel. It seems that the signal fits of GABA, 2HG and Glu+Gln are in competition to fit the total resonances in the 2-2.5 ppm region. For ‘Questionable’ data quality, the fit of Glu+Gln is dominating. The patients in this study received various treatments and almost all of them had surgical background. Therefore, data interpretation with respect to therapy and false positive rates is very complicated.Conclusion
It is feasible to assess reliable 2HG levels in patients with gliomas by integrating the 2HG MRS into clinical routine MRI examinations at 3 Tesla.Acknowledgements
We acknowledge funding form Eurostars Project E!12449 IMAGINE!.References
[1] Dang, L. et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462, 739–744 (2009).
[2] Yan, H. et al. IDH1 and IDH2 Mutations in Gliomas . N. Engl. J. Med. 360, 765–773 (2009).
[3] Choi, C. et al. 2-Hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat. Med. 18, 624–629 (2012).
[4] Andronesi, O. C. et al. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci. Transl. Med. 4, 116ra4 (2012).
[5] Branzoli, F. et al. Highly specific determination of IDH status using edited in vivo magnetic resonance spectroscopy. Neuro. Oncol. 20, 907–916 (2018).
[6] Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med.30(6):672-679 (1993).
Figures