3917
The combined value of DKI and DSC MRI in differentiating high-grade glioma recurrence from pseudoprogression1Department of Radiology, First Hospital of Shanxi Medical University, Taiyuan, China, 2Department of Radiology, Zhongda Hospital, Southeast University, Nanjing, China
Synopsis
To evaluate the diagnostic performance of DKI in differentiating glioma recurrence from pseudoprogression and the combined value of DKI and DSC MRI parameters. Tumor recurrence was confirmed to be associated with more tumor angiogenesis, greater nuclear atypia, and increased cell density, whereas pseudoprogression is characterized by radiation-induced vascular changes leading to vasodilation, edema, and increased capillary permeability. These resulted in a more complex structure of recurrent tumor than pseudoprogression. It is concluded DKI and DSC MRI may serve as imaging biomarker of treatment response by characterizing the heterogeneity of the microenvironment and newly formed immature blood vessels.
Introduction
There are pathophysiologic differences between glioma recurrence and pseudoprogression. Recent advances in MR techniques have made it possible to monitor tumors at the metabolic and microvascular levels. Relative cerebral blood volume (rCBV) derived from dynamic susceptibility contrast-enhanced (DSC) MRI, which is a recognized imaging biomarker of angiogenesis, is the most powerful single imaging classifier and convincing parameter for differentiating glioma recurrence from pseudoprogression. 1, 2 However, it is currently difficult to generate definitive conclusions that can confidently guide the use of the DSC technique in clinical practice. Diffusion kurtosis imaging (DKI), a non-invasive tool, can depict the non-Gaussian diffusion of water molecules and accurately characterize cellular density and tissue heterogeneity information. 3 We hypothesized that DKI may be accurate in making this differentiation and may have extra value for DSC MRI. The purpose of the present study was to analyze the value of DKI in discriminating glioma recurrence from pseudoprogression and to determine whether DKI combined with DSC MRI can improve differentiation compared with single use.Methods
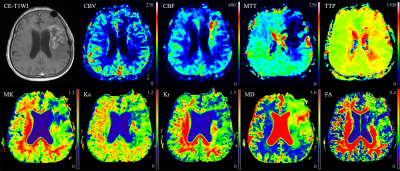
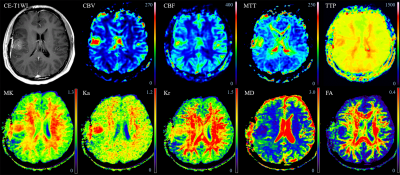
Thirty-four patients with high-grade gliomas developing new and/or increasing enhanced lesions within six months after surgery and chemoradiotherapy were retrospectively analyzed. All of the patients were confirmed to have recurrent glioma (n = 22) or pseudoprogression (n = 12) by reoperation or biopsy. The DKI and DSC MRI parameters were calculated based on the enhanced lesions on contrast-enhanced T1WI. ROC analysis was performed on significant variables to determine their diagnostic performance. Multivariate logistic regression was used to determine the best model for discrimination.Results
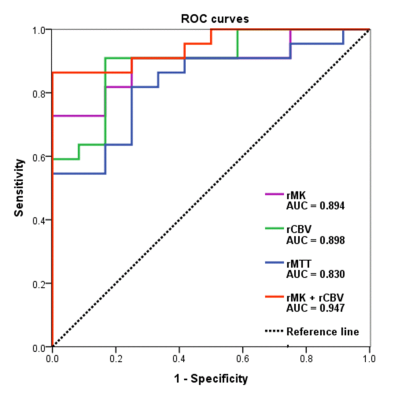
The mean relative mean kurtosis (rMK), rCBV, and relative mean transit time (rMTT) of glioma recurrence were higher than those of pseudoprogression (P < 0.001, P < 0.001, P = 0.001, respectively). The AUCs and diagnostic accuracy were 0.894 and 82.35% for rMK, 0.898 and 82.35% for rCBV, 0.830 and 79.41% for rMTT, respectively. A multivariate logistic regression model showed a significant contribution of rMK (P = 0.004) and rCBV (P = 0.001) as independent imaging classifiers for discrimination. The combined use of rMK and rCBV improved the AUC to 0.947 and the diagnostic accuracy to 85.29%.Discussion
rMK can be used to distinguish physiological differences between glioma recurrence and pseudoprogression. Tumor recurrence was confirmed to be associated with more tumor angiogenesis, greater nuclear atypia, and increased cell density, 4 whereas pseudoprogression is characterized by radiation-induced vascular changes leading to vasodilation, edema, and increased capillary permeability. 5 These resulted in a more complex structure of recurrent tumor than pseudoprogression. MK can reflect the difference of tumor internal heterogeneity, and the greater the parameter value of MK, the more complex the structure. 3, 6 Thus, MK is meaningful in terms of classification. DKI may serve as a novel imaging biomarker for differentiation by characterizing the heterogeneity of the microenvironment. Our study produced significantly higher rCBV and rMTT values in the recurrence group than in the pseudoprogression group. rCBV was the most accurate parameter in the application of DSC MRI to distinguish between recurrence and pseudoprogression, as in most previous studies. 1, 2, 7, 8 Newly formed immature blood vessels of recurrent tumors can produce increased blood volume and vascular permeability, as well as proportions of tumor cells, resulting in significantly higher rCBV values. 9, 10 The higher rCBV in tumors are associated with poor prognosis after radiotherapy, suggesting that a large number of tumor-induced angiogenesis are related to more active tumor growth. 11, 12 It is clear that increased contrast enhancement due to a disrupted blood-brain barrier may be affected by several factors, including acute changes after surgery or radiotherapy, chemotherapy, as well as MRI technical issues and administration of gadolinium. 13 ROC analysis showed that both rMK and rCBV demonstrated good classification ability and presented a similar diagnostic accuracy in distinguishing tumor recurrence from pseudoprogression. These data further promote the application of DKI to the clinical study of glioma recurrence and pseudoprogression, making it a possible and effective alternative to DSC MRI, especially when the administration of gadolinium is unavailable or the technique fails. Multivariate logistic regression analysis of the imaging parameters indicated that the best classification of tumor recurrence and pseudoprogression was achieved using two parameters, namely, rMK and rCBV. The combined use of the two parameters improved the diagnostic performance compared with either parameter alone, and is probably related to the fact that changes associated with the treatment of lesions are complex and variable. 13 Therefore, mean rMK and rCBV may be influenced by the parameters from both tumoral and nontumoral components. 14Conclusion
DKI is a potential non-invasive imaging biomarker of response that may help differentiate glioma recurrence from pseudoprogression and may have an equivalent predictive value as DSC MRI. The combination of rMK and rCBV may be used to improve diagnostic performance in assessing treatment response.Acknowledgements
No acknowledgement found.References
1. Blasel S, Zagorcic A, Jurcoane A, et al. Perfusion MRI in the Evaluation of Suspected Glioblastoma Recurrence. J Neuroimaging. 2016;26:116-123.
2. Khalifa J, Tensaouti F, Chaltiel L, et al. Identification of a candidate biomarker from perfusion MRI to anticipate glioblastoma progression after chemoradiation. Eur Radiol. 2016;26:4194-4203.
3. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53:1432-1440.
4. Mahase S, Rattenni RN, Wesseling P, et al. Hypoxia-Mediated Mechanisms Associated with Antiangiogenic Treatment Resistance in Glioblastomas. Am J Pathol. 2017;187:940-953.
5. Jensen RL. Brain tumor hypoxia: tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a therapeutic target. J Neurooncol. 2009;92:317-335.
6. Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010;23:698-710.
7. Patel P, Baradaran H, Delgado D, et al. MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: a systematic review and meta-analysis. Neuro Oncol. 2017;19:118-127.
8. Nael K, Bauer AH, Hormigo A, et al. Multiparametric MRI for Differentiation of Radiation Necrosis From Recurrent Tumor in Patients With Treated Glioblastoma. AJR Am J Roentgenol. 2018;210:18-23.
9. Barajas RF Jr, Chang JS, Segal MR, et al. Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2009;253:486-496.
10. Yun TJ, Park CK, Kim TM, et al. Glioblastoma treated with concurrent radiation therapy and temozolomide chemotherapy: differentiation of true progression from pseudoprogression with quantitative dynamic contrast-enhanced MR imaging. Radiology. 2015;274(3):830-40.
11. Cao Y, Tsien CI, Nagesh V, et al. Survival prediction in high-grade gliomas by MRI perfusion before and during early stage of RT [corrected]. Int J Radiat Oncol Biol Phys. 2006;64:876-885.
12. Kong Z, Yan C, Zhu R, et al. Imaging biomarkers guided anti-angiogenic therapy for malignant gliomas. Neuroimage Clin. 2018;20:51-60.
13. Ellingson BM, Wen PY, Cloughesy TF. Modified Criteria for Radiographic Response Assessment in Glioblastoma Clinical Trials. Neurotherapeutics. 2017;14:307-320.
14. Toh CH, Castillo M, Wong AM, et al. Primary cerebral lymphoma and glioblastoma multiforme: differences in diffusion characteristics evaluated with diffusion tensor imaging. AJNR Am J Neuroradiol. 2008;29:471-475.
Figures