3908
Twofold Improved Tumor-to-Brain Contrast using a Novel T1 Relaxation-Enhanced Steady-State (T1RESS) Technique
Robert R Edelman1,2, Nondas Leloudas3, Jianing Pang4, Julian Bailes5, Ryan Merrell6, and Ioannis Koktzoglou3,7
1Radiology, NorthShore University HealthSystem, EVANSTON, IL, United States, 2Feinberg School of Medicine, Northwestern University, Chicago, IL, United States, 3Radiology, NorthShore University HealthSystem, Evanston, IL, United States, 4Siemens Medical Solutions USA, Chicago, IL, United States, 5Neurosurgery, NorthShore University HealthSystem, Evanston, IL, United States, 6Medicine, NorthShore University HealthSystem, Evanston, IL, United States, 7Pritzker School of Medicine, University of Chicago, Chicago, IL, United States
1Radiology, NorthShore University HealthSystem, EVANSTON, IL, United States, 2Feinberg School of Medicine, Northwestern University, Chicago, IL, United States, 3Radiology, NorthShore University HealthSystem, Evanston, IL, United States, 4Siemens Medical Solutions USA, Chicago, IL, United States, 5Neurosurgery, NorthShore University HealthSystem, Evanston, IL, United States, 6Medicine, NorthShore University HealthSystem, Evanston, IL, United States, 7Pritzker School of Medicine, University of Chicago, Chicago, IL, United States
Synopsis
We describe a novel class of steady-state pulse sequence called T1 Relaxation-Enhanced Steady-State (T1RESS). Several versions have been implemented including: (a) “bright blood” balanced T1RESS (bT1RESS), and (b) “dark blood” unbalanced T1RESS (uT1RESS). There is also a two-echo Dixon version for fat/water separation. In a brain tumor study, contrast-enhanced T1RESS demonstrated a remarkable two-fold improvement in tumor-to-brain contrast as well as enhanced SNR compared with standard neuroimaging techniques, including MP-RAGE and T1 SPACE. Our initial results suggest that T1RESS has great potential for oncological imaging in the brain, and with further development in other organ systems as well.
INTRODUCTION
Brain metastases occur in 20 to 40% of patients with cancer and are the most frequent intracranial tumor in these patients. Early detection and differentiation from other types of brain pathology is essential for optimal patient management and therapy. While contrast-enhanced magnetic resonance imaging (MRI) is the cornerstone for brain tumor diagnosis and treatment planning, standard neuroimaging techniques have remained fairly static over the past decade. Available pulse sequences include 2D fast spin-echo and 3D implementations such as variable flip angle fast spin-echo (3D-VFA-FSE, also called SPACE, CUBE or VISTA), as well as 2D and inversion-prepared 3D spoiled GRE (3D IR-SPGRE, also called MP-RAGE). Despite its excellent SNR efficiency and utility for cardiac MRI, balanced SSFP (bSSFP) is primarily reserved for niche applications in the brain, in part due to the dependence of bSSFP signal on the T2/T1 ratio which makes it insensitive to Gd enhancement. In addition, CSF appears bright and the images are prone to off-resonance artifacts near the paranasal sinuses and skull base.We have developed a novel class of steady-state pulse sequence called T1 Relaxation-Enhanced Steady-State (T1RESS) (1). In addition to providing excellent T1 contrast and SNR efficiency, it provides the unique capability to toggle intravascular signal on and off in order to improve tumor conspicuity. To test its clinical utility, T1RESS was compared with standard neuroimaging techniques in a series of patients with brain tumors.
METHODS
The prototype T1RESS pulse sequence is illustrated in Fig. 1. T1RESS introduces a flexible degree of T1 weighting while maintaining excellent SNR efficiency by repeatedly applying additional non-spatially selective partial saturation contrast-modifying (CMα) RF pulses throughout the duration of the echo train. The CMa RF pulses can be adjusted independently of the imaging RF pulses. The amount of T1 weighting can be changed as needed by varying the values for the CMα flip angle and TR, with larger flip angles and shorter TR resulting in more T1 weighting. Several variants of T1RESS have been implemented. One version, which we call “balanced” T1RESS (bT1RESS) uses a readout in which the gradient moments are fully balanced to make contrast-enhanced blood vessels appear bright. A second “unbalanced” version (uT1RESS) renders blood vessels dark by using a steady-state readout in which the gradients are unbalanced. In addition, a two-echo Dixon uT1RESS version permits fat/water imaging.A proof-of-concept study of patients with suspected or known brain tumors was approved by the hospital institutional review board. Contrast-enhanced MRI of the brain was performed at 3 Tesla (MAGNETOM Skyra and MAGNETOM Skyrafit, Siemens Healthcare, Erlangen Germany) in 54 adult subjects (ages 19 - 88 years, 27 female) with suspected or known brain tumors. The range of tumor pathology included meningioma, astrocytoma, glioblastoma and metastatic disease. For contrast-enhanced MRI of the head, 0.1 mmol/kg of gadobutrol (Bayer, Berlin, Germany) was administered intravenously followed by standard-of-care 2D fast spin-echo, 3D IR-SPGRE and/or T1-weighted 3D-VFA-FSE. Typical values for the CMα flip angle and TR were ≈75o and ≈400 milliseconds (msec), respectively.
RESULTS
For both the balanced and unbalanced versions of T1RESS, the periodic application of CMα RF pulses was essential for generating T1 contrast and to suppress the signal intensity of cerebrospinal fluid. The two-echo Dixon uT1RESS implementation provided robust fat/water separation over the entire field-of-view with no evidence of fat/water swapping. The uniform fat suppression provided by the Dixon version was especially helpful for evaluation of the orbits and neck regions. With uT1RESS, enhancing tumors appeared particularly conspicuous against a background in which blood vessels along with non-enhancing tissues all appeared relatively dark. With this method, we found that even minute metastatic tumor deposits that were difficult to distinguish from small blood vessels using standard imaging techniques could be unambiguously identified (Fig. 2). Moreover, banding artifacts from off-resonance effects were absent.Comparing uT1RESS with 3D IR-SPGRE, the respective values for mean CNR of brain tumors were 164.37 ± 97.21 vs. 100.45 ± 86.45, p = 7.62x10-6, the values for mean tumor-to-brain contrast were 1.69 ± 0.89 vs. 0.82 ± 0.55, p = 5.14x10-8. Comparing uT1RESS with T1 3D-VFA-FSE, the respective values for mean CNR of brain tumors were 180.32 ± 92.34 vs. 60.34 ± 54.88, p = 8.75x10-7 and the respective values for mean tumor-to-brain contrast were 1.76 ± 0.76 vs. 0.89 ± 0.43, p = 7.95x10-7.
DISCUSSION AND CONCLUSION
T1RESS represents a fundamental redesign of the traditional steady-state pulse sequence architecture. This novel MRI method enables the flexible modulation of T1 weighting and provides the unique feature that intravascular signals can be toggled on and off in contrast-enhanced scans. We hypothesize that the combination of twofold improved tumor-to-background contrast and flexible control over intravascular signal has the potential to make T1RESS a valuable tool for the evaluation of brain tumors in general, and, more specifically, to provide a clinically significant boost in accuracy for the detection and characterization of metastatic disease. In addition to its benefits for imaging brain tumors, initial studies suggest it could prove useful for a variety of other brain disorders such as multiple sclerosis, as well as for tumors involving other organ systems.Acknowledgements
FUNDING SOURCES: NIH grants R01 HL137920, R01 HL130093 and R01 EB027475.References
1. Edelman R, Leloudas N, Pang J, Bailes J, Merrell R, Koktzoglou I. Twofold improved tumor-to-brain contrast using a novel T1 relaxation-enhanced steady-state (T1RESS) MRI technique. Sci Adv. 2020;6(44). Epub 2020/10/30. doi: 10.1126/sciadv.abd1635. PubMed PMID: 33115747.Figures
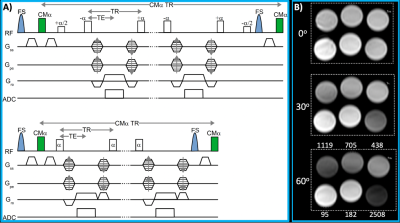
Fig. 1. A) T1RESS pulse sequence using balanced (top) and unbalanced (bottom) steady-state readouts. A non-spatially selective contrast-modifying RF pulse (CMα) is applied periodically over the entire duration of the echo train to introduce an arbitrary amount of T1 weighting. B) Phantom consisting of serial dilutions of gadobutrol imaged with bT1RESS using CMα values of 0o, 30o and 60o. Note that there is negligible T1 contrast for a CMα flip angle of 0o, but substantial T1 contrast is apparent as the flip angle is increased to 60o.
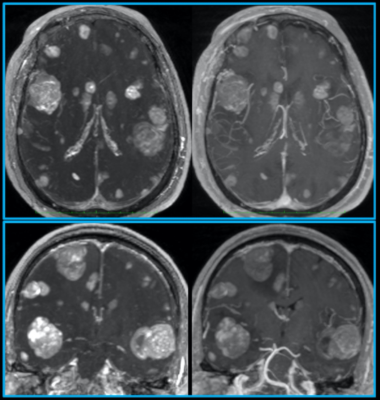
Fig. 2. Pulse sequence comparisons after contrast administration in a patient with metastatic melanoma. 10-mm thick axial (top) and coronal (bottom) maximum intensity projections are shown to highlight differences in lesion visibility for uT1RESS (left) and 3D spoiled GRE (right). Small metastatic lesions are much better visualized using uT1RESS than with 3D spoiled GRE. The combination of twofold increased tumor-to-background contrast, improved CNR and suppression of intravascular signals with uT1RESS is helpful to unambiguously identify small metastases.