3905
A pilot study of 18F-PSMA PET/CT or PET/MRI and ultrasound fusion targeted prostate biopsy for intra-prostatic PET-positive lesions1Department of Nuclear Medicine, Chinese PLA General Hospital, Beijing, China, 2Department of Urology Surgery, Chinese PLA General Hospital, Beijing, China, 3MR collaborations, Diagnostic Imaging, Siemens Healthcare, Shanghai, China, 4MR Education, Customer Services, Siemens Healthcare, Erlangen, Germany
Synopsis
The purpose of this study is to evaluate the feasibility and diagnostic performance of 18F-PSMA PET/CT-ultrasound (PET/CT-US) or PET/MRI-ultrasound (PET/MRI-US) fusion targeted biopsy for intra-prostatic PET-positive lesions. 55 candidates were prospectively enrolled for PET/CT-US or PET/MRI-US fusion targeted biopsies at solitary PET-positive lesions. A total of 178 core biopsies were performed on 55 patients. 146 biopsy cores (82.0%, 146/178) from 51 (92.7%, 51/55) patients were positive for prostate cancer; 47 (85.5%, 47/55) were clinically significant prostate cancer. 18F-PSMA PET/CT-US or PET/MRI-US fusion-targeted prostate biopsy are feasible for prostate cancer diagnosis and has high detection rate of clinically significant prostate cancer.
Background and Purpose
Prostate cancer remains one of the most common male malignancies worldwide. Systematic 12-core transrectal ultrasound-guided prostate biopsy and histopathology are the most commonly used techniques for the diagnosis of prostate cancer before radical prostatectomy [1]. However, systematic biopsies are invasive, painful, and prone to potential complications. In addition, this conventional approach is poor at sampling the anterior, midline, and apex of the prostate, which leads to the underdiagnosis of patients with clinically significant prostate cancer.Clinicians are constantly exploring new methods, such as direct in-bore MRI guidance and image fusion guidance targeted prostate biopsy, to improve the detection of clinically significant prostate cancer and reduce the number of biopsy procedures and associated complications [2]. PSMA is a type II transmembrane glycoprotein with enzymatic carboxypeptidase activity. A previous study showed that 18F-labeled PSMA provides better image quality and the ability to display small lesions [3]. The purpose of this study was to evaluate the feasibility and diagnostic performance of prostate-specific membrane antigen (PSMA) based 18F-PSMA PET/CT-ultrasound (PET/CT-US) or PET/MRI-ultrasound (PET/MRI-US) fusion targeted biopsy for intra-prostatic PET-positive lesions.
Methods
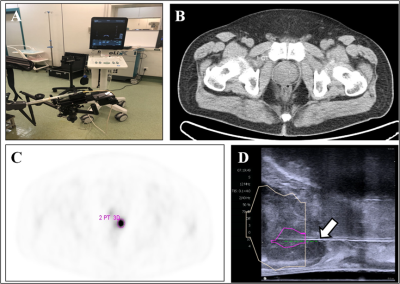
From April 2018 to November 2019, we prospectively enrolled 55 candidates to perform PET/CT-US or PET/MRI-US fusion targeted biopsies for solitary PET-positive prostate lesions (2-4 cores/lesion). PET/CT was performed from the ears to the upper thigh on a Siemens Biograph 64 operating in 3D emission mode. PET/MRI was performed on a hybrid PET/MRI scanner (Biograph mMR, Siemens Healthcare, Erlangen, Germany). 18F-PSMA PET/CT-US or PET/MRI-US fusion targeted prostate biopsy for the intra-prostatic PET-positive lesions were performed with the BK Predictive Fusion prostate biopsy system (BK Medical Technology Shanghai Co., Ltd).The positive rates of prostate cancer based on patients and biopsy cores were calculated respectively. In our study, clinically significant prostate cancer was defined as the presence of a single biopsy core indicating disease of Gleason score 3+4 (Gleason sum of 7) or greater (the Gleason score is composed of a primary grade plus a secondary grade). With reference to the pathological results of biopsy cores, the MR signal characteristics in the area of the PET-positive lesion were analyzed for the patients who underwent PET/MR.
Results
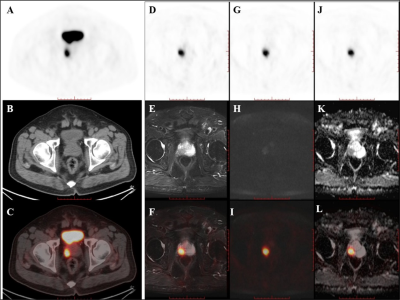
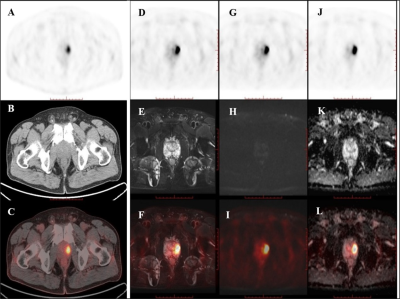
A total of 178 core biopsies were performed on the 55 patients. 146 biopsy cores (82.0%, 146/178) from 51 (92.7%, 51/55) patients were positive for prostate cancer; 47 (85.5%, 47/55) were clinically significant prostate cancer.Among the 55 patients, 32 patients received PET/CT examinations along, 14 patients received PET/MRI examinations along, and 9 patients underwent both PET/CT and PET/MRI scans sequentially within two hours. Of the 14 patients examined using only 18F-PSMA PET/MR, 13 were diagnosed with clinically significant cancer, and 1 was diagnosed with clinically insignificant cancer. All of these 14 patients showed abnormal MR signals (low T2 signal, high DWI signal, decreased ADC value) in the area of PET-positive lesions. Of the 9 patients that underwent successive examinations of 18F-PSMA PET/CT and PET/MRI, the 7 patients with prostate cancer showed abnormal MR signal in the area of the PET-positive lesion while the other 2 patients with prostatic hyperplasia and prostatitis showed normal MR signal in the area of the PET-positive lesion.
Discussion
This preliminary study demonstrates that for patients with clinical suspicion of prostate cancer and 18F-PSMA PET-positive lesions, using PET/CT-US or PET/MRI-US fusion-targeted prostate biopsy had a high detection rate of clinically significant prostate cancer. Furthermore, PET/MR was able to identify the false positive lesions by PSMA PET. To our knowledge, this is the first study to explore the feasibility and diagnostic performance of PSMA PET/CT-US and PET/MRI-US fusion targeted prostate biopsy.The results of our study showed that fusion of 18F-PSMA PET/CT or PET/MRI with ultrasound is beneficial and feasible for guiding targeted prostate biopsy. In addition, this preliminary result indicates that 18F-PSMA PET/CT-US or PET/MRI-US fusion targeted prostate biopsy may be a good way to reduce over-diagnosis of clinically insignificant prostate cancer and improve detection of clinically significant cancer. This method allows urologists to progress from blind, systematic biopsies to biopsies that are mapped, targeted, and tracked. More rigorous and comprehensive studies should be designed to prove the clinical value of PET/CT-US and PET/MRI-US fusion targeted prostate biopsies.
There are limitations to our study. Firstly, the sample size is small. Considering the limited number of cases in this study, we were unable to compare the diagnostic value between PET/CT-US and PET/MR-US. Secondly, this study only used 18F-PSMA PET/CT-US and PET/MR-US guided biopsy for targeted PET-positive lesions; therefore, we were unable to directly compare them with the systematic prostate biopsies.
Conclusion
In this study, 18F-PSMA PET/CT-US or PET/MRI-US fusion-targeted prostate biopsy proved to be feasible for prostate cancer diagnosis due to its high detection rate of clinically significant prostate cancer. PET/MR can rule out some false PET-positive lesions, which may potentially reduce unnecessary prostate biopsies. For patients with pacemakers or claustrophobia, 18F-PSMA PET/CT-US guided prostate biopsy remains a good alternative.Acknowledgements
No acknowledgement found.References
[1] Thompson I, Thrasher JB, Aus G et al (2007) Guideline for the Management of Clinically Localized Prostate Cancer: 2007 Update. Journal of Urology 177:2106-2131
[2] Wegelin O, Exterkate L, van der Leest M et al (2019) Complications and Adverse Events of Three Magnetic Resonance Imaging-based Target Biopsy Techniques in the Diagnosis of Prostate Cancer Among Men with Prior Negative Biopsies: Results from the FUTURE Trial, a Multicentre Randomised Controlled Trial. Eur Urol Oncol 2:617-624
[3] Dietlein M, Kobe C, Kuhnert G et al (2015) Comparison of [18F]DCFPyL and [68Ga]Ga-PSMA-HBED-CC for PSMA-PET Imaging in Patients with Relapsed Prostate Cancer. Molecular Imaging and Biology 17:575-584
Figures