Lihua Chen1, Ailian Liu1, Yan Guo2, and Xin Li2
1The First Affiliated Hospital of DaLian Medical University, Dalian, China, 2GE Healthcare, China, Beijing, China
Synopsis
DWI might be used as a biomarker for tumor aggressiveness, and
various reports have been made on using DWI in distinguishing PCa from BPH. The primary parameter of DWI, ADC must be obtained with a high b value DWI, to limit
the perfusion effect. The term radiomics has attracted increased attention in
recent years, which was presented by Lambin in 2012. The aim of this study was
to establish and evaluate the efficiency of radiomics model in distinguishing
PCa from BPH based on DWI sequence and clinical
information, and to compare the efficiency of ROI sketched by two different
methods.
Introduction
Prostate
cancer (PCa) is the second most common cancer among males [1].
Multi-contrast MR imaging has become widely used in risk stratification and
treatment planning [2]. Diffusion-weighted imaging(DWI) might be
used as a biomarker for tumor aggressiveness, and various reports have been
made on using DWI in distinguishing PCa from benign prostatic hyperplasia (BPH)[3,4].
However, the primary parameter of DWI, apparent diffusion coefficient (ADC)
must be obtained with a high b value DWI, to limit the perfusion effect. While
the contribution of perfusion effect to the ADC values and T2 effect on trace
images decrease at high b values which led to impact on our diagnosing. The
term radiomics has attracted increased attention in recent years, which was
presented by Lambin in 2012 [5], and it is the process of the
conversion of medical images into high-dimensional, mineable data via
high-throughput extraction of quantitative features, followed by subsequent
data analysis for decision support [6,7]. Radiomics, which allows
the investigation of multiple imaging features in parallel, can provide a
combination of features [8].
The aim of this study was to establish and evaluate the efficiency of
radiomics model in distinguishing PCa from BPH based on diffusion-weighted
imaging (DWI) sequence and clinical information, and to compare the efficiency
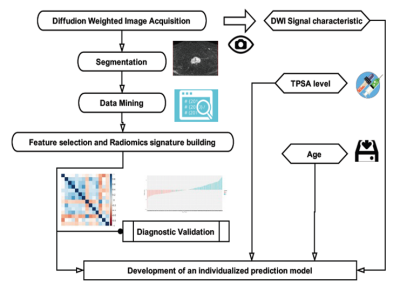
of ROI sketched by two different methods.Materials and Methods
The diagnostic model was developed in a training
dataset that consisted of 200 patients with PCa or BPH, and data was
gathered from January 2010 to March 2017. Radiomic features were
extracted from DWI of PCa and BPH, here we extracted features from the whole prostate gland (plan A) and the focus only (plan B), respectively. Logistic regression model was used for
radiomics signature building and to develop the diagnostic model which incorporated
the radiomics signature with patient age, DWI signal characteristics, and
independent clinicpathologic risk factors, and this was presented with radiomics
nomograms. The performance of each nomogram in plan A and B was
assessed with respect to its calibration, discrimination, and clinical
usefulness. An independent testing dataset contained 60 consecutive patients
from March 2017 to October 2017. The workflow was shown as Figure 1.Results
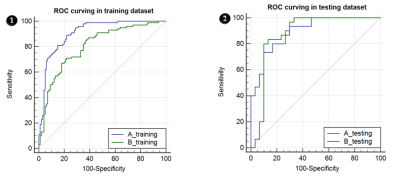
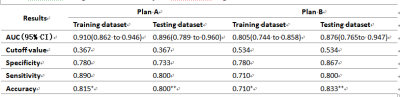
The radiomics signatures, which consisted of 16
selected features and 14 selected features, respectively in plan A and
plan B, were statistically different
(P < 0.001) between PCa and BPH group both in training and testing dataset. AUC
were 0.896 and 0.876, specificity were 0.733 and 0.867, sensitivity were 0.800
and 0.800,accuracy rate was 80.0% and 83.3% in testing dataset, respectively in
plan A and B (Figure 2, Table 1). Predictors contained in the individualized diagnostic
nomograms included the radiomics signature, patient age, DWI signal
characteristics, and total prostate specific antigen level. The models showed good discrimination in
which plan A was better than plan B, Calibration Curve and Decision curve analysis demonstrated that
the radiomics nomograms were clinically useful.Conclusion
The newly established comprehensive model is
efficient in clinical distinguishing PCa from BPH, in which the method sketching
the whole prostate gland may have a better prospect for prostate radiomics
study. Acknowledgements
No acknowledgement found.References
[1] World Cancer Research
Fund International/American Institute for Cancer Research Continuous Update
Project Report: Diet, Nutrition, Physical Activity, and Prostate Cancer. 2014.
[2] Larissa J
Vos, Michele Janoski, Keith Wachowicz,et al. Role of serial multiparametric
magnetic resonance imaging in prostate cancer active surveillance. World
Journal of Radiology. 2016, 8(4):410-418.
[3] Kilinç R,
Doluoglu OG, Sakman B, et al. The Correlation between Diffusion-Weighted
Imaging and Histopathological Evaluation of 356 Prostate Biopsy Sites in
Patients with Prostatic Diseases. ISRN Urology. 2012:252846.
[4] Liney GP,
Holloway L, Al Harthi TM, et al. Quantitative evaluation of diffusion-weighted
imaging techniques for the purposes of radiotherapy planning in the prostate.
British Institute of Radiology. 2015, 88(1049):20150034.
[5] Lambin P, Rios-velazquez
E, Leijenaar R, et al.Radiomics: extracting more
information from medical imagesusing advanced feature analysis. European
Cancer, 2012,48(4): 441-446.
[6]
Aerts HJ, Velazquez ER, Leijenaar RT, et al.Decoding tumour phenotype by
noninvasive imagingusing a quantitative radiomics approach. Nat Commun5:4006,
2014 [Erratum: Nat Commun 5:4644,2014].
[7].
Gillies RJ, Kinahan PE, Hricak H: Radiomics:Images are more than pictures, they
are data.Radiology. 2016, 278:563-577.
[8]
Huang YQ, Liang CH, He L, et al. Development and Validation of a Radiomics
Nomogram forPreoperative Prediction of Lymph Node Metastasis inColorectal
Cancer. Journal of Clinical Oncology. 2016, 34(18):2157-2164.