3889
Preliminary Exploration of Expanding the Target Population of VI-RADS - Could Patients After Treatment Benefit from It?1Radiology, Zhongshan Hospital Fudan University, Shanghai, China, 2Urology, Zhongshan Hospital Fudan University, Shanghai, China, 3MR Research, GE Healthcare, MR Research, Beijing, China
Synopsis
In this study, we expanded the target population by including patients after treatment and with recurrent bladder cancer to further evaluate the diagnostic performance of VI-RADS. The diagnostic performances of VI-RADS were good in all subgroups including the primary, post-treatment and recurrence groups (AUC > 0.90). No significant differences were observed in diagnostic efficacy of VI-RADS between the primary group and the post-treatment group, and between the primary group and the recurrence group. Overall, this study demonstrated that VI-RADS can be considered an effective preoperative imaging staging tool for a wider range of bladder cancer patients.
Abstract
IntroductionVesical Imaging-Reporting and Data System (VI-RADS) has been shown to have good diagnostic efficacy in differentiating muscular and non-muscular invasive bladder cancer1,2. However, the original intention of VI-RADS and previous studies have only focused on the target population of patients with primary bladder cancer1-3. Post-treatment patients, including those with recurrence, were not included in the evaluation. However, bladder cancer has a high risk of recurrence and progression4,5, and recurrent patients are also faced with staging problems in deciding which surgery to have next or systemic treatment. Therefore, we expanded the target population by including patients after treatment into the cohort, and further evaluated the diagnostic performance of VI-RADS. The premise of using VI-RADS is that the bladder space occupying is bladder cancer. We excluded patients who had localized thickening of the bladder wall after surgery, as it is difficult to differentiate between inflammatory and neoplastic lesions3,6. The remaining patients, including post-treatment patients, were included prospectively to assess the scope of VI-RADS application.
Materials and Methods
From January 2020 to October 2020, we prospectively performed preoperative multiparametric magnetic resonance imaging (mpMRI) on patients with suspected bladder cancer, including post-treatment patients. All data were acquired on a 3T MRI scanner (DiscoveryTM MR750, GE Healthcare, Milwaukee, WI). According to VI-RADS, T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and dynamic contrast-enhanced (DCE) imaging were obtained. The entire cohort was divided into the primary bladder cancer group and the post-treatment group, and the recurrence group was separated from the post-treatment group. VI-RADS score were performed on the whole group and subgroups, respectively. A score of 3 or greater was defined as imageological muscle infiltration. The diagnostic efficacy was assessed by calculating the sensitivity, specificity, positive (PPV) and negative predictive values (NPV) for the whole cohort of patients and all subgroups, using postoperative pathological reports as standard reference. The receiver operating characteristic (ROC) curve analysis was performed, and the area under the curve (AUC) was used to assess the performance of VI-RADS score for predicting muscle-invasive tumor in each group. Z test was performed to compare the difference of AUC between groups. Two-tailed P value less than 0.05 was considered significant. The study design is shown in Figure 1.
Results
A total of 73 patients were enrolled. For the whole group, a score of 3 or greater for VI-RADS to predict muscle invasive tumors resulted in the sensitivity, specificity, PPV, and NPV of 90.0% (95% confidence interval [CI]: 68.3–98.8), 86.8% (95% CI: 74.7–94.5), 72.0% (95% CI: 50.6–87.9) and 95.8% (95% CI: 85.7–99.5), respectively. The AUC value was 0.945 (95% CI: 0.865–0.985). For the primary group, the sensitivity, specificity, PPV, and NPV were 87.5% (95% CI: 47.3–99.7), 91.2% (95% CI: 76.3–98.1), 70.0% (95% CI: 34.8–93.3), and 96.9% (95% CI 83.8–99.9), respectively. For the post-treatment group, the sensitivity, specificity, PPV, and NPV were 91.7% (95% CI: 61.5–99.8), 79.0% (95% CI: 54.4–93.9), 73.3% (95% CI: 44.9–92.2), and 93.7% (95% CI 69.8–99.8), respectively. The AUC of the primary group was 0.936 (95% CI: 0.815–0.988) and the AUC of the post-treatment group was 0.947 (95% CI: 0.803–0.995). For the recurrence group, the sensitivity, specificity, PPV, and NPV were 88.9% (95% CI: 51.8–99.7), 85.7% (95% CI: 57.2–98.2), 80.0% (95% CI: 44.4–97.5), and 92.3% (95% CI: 64.0–99.8), respectively. The AUC value was 0.944 (95% CI: 0.763–0.997). No significant differences were observed in AUC between the primary group and the post-treatment group (p = 0.870), and in AUC between the primary group and the recurrence group (p = 0.912). Sensitivity, specificity and ROC results in all groups are summarized in Figure 2.
Discussion
In the entire cohort including patients with bladder cancer after treatment, VI-RADS still performed well in identifying muscle infiltration. In the primary group, post-treatment group and recurrence group, all AUC values of VI-RADS were greater than 0.90. Our results demonstrated that there were no statistically significant differences in diagnostic efficacy of VI-RADS between the primary group and the post-treatment group, and between the primary group and the recurrence group. These results indicated that that post-treatment changes in the bladder wall do not completely limit the applicability of VI-RADS for these patients.
Conclusions
Our results showed that VI-RADS does not need to be limited to patients with incipient BC. Patients after treatment and with recurrent disease may benefit from it as well under certain conditions. Overall, this study demonstrated that VI-RADS can be considered an effective preoperative imaging staging tool for a wider range of BC patients and should be investigated further.
Acknowledgements
No acknowledgement found.References
1. Thoeny HC, Bellin M, Comperat E, Thalmann GN. Vesical Imaging-Reporting and Data System (VI-RADS): Added Value for Management of Bladder Cancer Patients? Eur Urol 2018; 74:307-308.
2. Wang H, Luo C, Zhang F, et al. Multiparametric MRI for Bladder Cancer: Validation of VI-RADS for the Detection of Detrusor Muscle Invasion. Radiology 2019; 291:668-674.
3. Panebianco V, Narumi Y, Altun E, et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting And Data System). Eur Urol 2018; 74:294-306.
4. Kluth LA, Black PC, Bochner BH, et al. Prognostic and Prediction Tools in Bladder Cancer: A Comprehensive Review of the Literature. Eur Urol 2015; 68:238-253.
5. Gontero P, Sylvester R, Pisano F, et al. Prognostic Factors and Risk Groups in T1G3 Non–Muscle-invasive Bladder Cancer Patients Initially Treated with Bacillus Calmette-Guérin: Results of a Retrospective Multicenter Study of 2451 Patients. Eur Urol 2015; 67:74-82.
6. Kim B, Semelka RC, Ascher SM, Chalpin DB, Carroll PR, Hricak H. Bladder tumor staging: comparison of contrast-enhanced CT, T1- and T2-weighted MR imaging, dynamic gadolinium-enhanced imaging, and late gadolinium-enhanced imaging. Radiology 1994; 193:239-245.
Figures
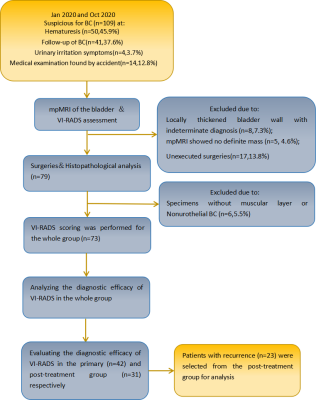
Fig. 1-Design of the study. BC= bladder cancer; mpMRI = multiparametric magneticresonance imaging; VI-RADS = Vesical Imaging Reporting and Data System.