3883
Multi-parametric functional and structural assessment of the placenta at late gestational ages using MRI1Sagol Brain Institute, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel, 2School of Computer Science and Engineering, The Hebrew University of Jerusalem, Jerusalem, Israel, 3Laboratory of FMRI Technology (LOFT), USC Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 4Division of Pediatric Radiology, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel, 5Sackler Faculty of Medicine & Sagol School of Neuroscience, Tel Aviv University, Tel Aviv, Israel, 6Ultrasound Unit, Lis Maternity Hospital, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel
Synopsis
Early and accurate placental insufficiency assessment is of a great importance. Here we comprehensively assessed placental perfusion and structure, and fetal brain and body volumes, in normal fetuses and in cases with fetal growth restriction (FGR) caused by placental insufficiency, at late gestational ages (GA=31-34 weeks). Increased Placental Blood Flow and Arterial Transit Time were detected in normal cases compared to earlier GA reported previously. Significant differences were found in several placental and fetal characteristics between normal and FGR cases. This study emphasizes the importance of multi-parametric assessment of fetuses to enable identification of high risks for FGR.
Introduction
The placenta is an essential organ for normal development of the fetus. Placental insufficiency, i.e. impairment of the placental function, is most frequently represented by fetal growth restriction (FGR), and carries an increased risk for neonatal, childhood and adulthood morbidity and mortality1. Several placental functional and structural parameters are known to characterize normal placental development and placental insufficiency. Uterine Artery Doppler Ultrasound, which is the method of choice in obstetrics, providing indirect information regarding blood flow within the umbilical cord, detects abnormal flow in cases with placental insufficiency2. Placental volumes were shown to increase with gestational age (GA), while smaller placental volumes and marginal umbilical cord insertion were found to be associated with abnormal placental function. Using advanced MRI methods, several studies characterized changes in placental perfusion using Arterial-Spin-Labeling (ASL) with GA, reporting inconsistent results3-5. Despite these methodological advancements and knowledge, perfusion it is still difficult to diagnose placental insufficiency before it affects the fetus6. The aims of this study were: (1) To provide normal placental perfusion values using ASL in late GA (31-34 weeks). Currently, there are only limited studies on human placenta using ASL, that show inconsistent results on placental perfusion changes with GA. (2) To use a multi-parametric approach to assess placental perfusion and structure while quantifying fetal development in normal developing fetuses and in a subgroup of fetuses diagnosed with FGR caused by placental insufficiency. We hypothesize that using multi-parametric approach will improve placental insufficiency assessment.Methods
Subjects: Seventeen fetuses at late GA (31-38 weeks) were included in this study. Of this cohort, twelve cases were normally developed with no evidence of placental abnormality, and five cases were diagnosed with FGR caused by placental insufficiency.MRI acquisition: Data was acquired on 3T Siemens MR magnets (PRISMA/Skyra).
Placental perfusion assessment: A multidelay 3D inner-volume GRASE free-breathing pCASL sequence, as reported by Shao et al.5 with three postlabeling delays (PLDs) of 1000, 1500 and 2000 msec, was used. High-resolution T2-weighted MRI (HASTE) with the same coverage as pCASL were acquired to visualize the placental anatomy.
Placental and fetal structural assessment: A total fetal body and placenta volumetric scan (TRUFI) was acquired in order to segment and assess the placental volume, fetal body volume and umbilical cord insertion location. Fetal brain volumetric scan (TRUFI) was used to assess fetal brain volume.
Image analysis: Placental blood flow (PBF) and arterial transit time (ATT) were calculated using a model fitting according to 5. Placental volume of interest (VOI) was drawn manually based on T2-weighted GRASE control images, and mean values of PBF and ATT were calculated for each VOI. The volume of the placenta was manually extracted, and the volumes of the fetal body and brain were obtained using automatic segmentation methods7. Fetal growth percentiles were assessed based on 8 for fetal body and based on 9 for fetal brain volumes. Volume to weight transformation was obtained by multiplying in a specific gravity of 1.13, based on 10. Umbilical-cord insertion location was quantified by the relative distance of the insertion site from the center similar to 11 while taking into account the location in two planes.
Statistical analysis: Differences between normal and FGR were assessed using paired t-test.
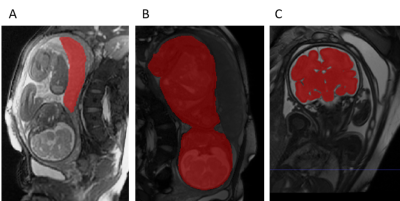
Radiological assessment and histopathology of the placentas: All five FGR cases were assessed by an expert pediatric neuro-radiologist. Three FGR placentas underwent histopathological assessment to confirm imaging results.
Results and Discussion
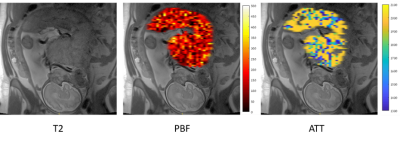
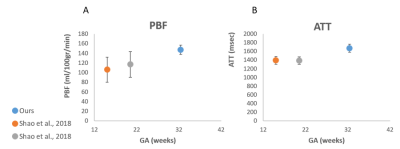
PBF and ATT maps were calculated for all placentas. Representative results for one placental slice, are demonstrated in Figure 1.Normal placental perfusion assessment: Normal placental perfusion values were: PBF=147± 9.4 ml/100g/min; ATT=1671.58± 93.9 msec, at mean GA=32.4± 0.6 weeks. Compared to previous studies using the same method at earlier GA, we detected higher PBF and ATT values, which is consistent with previous ultrasound study12. A plot of the mean PBF and ATT values of our cohort, in comparison to two time points of earlier GA5 is shown in Figure 2 A and B, respectively.
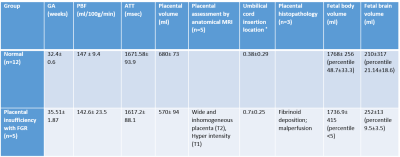
Placental and fetal functional and structural assessment: Perfusion, volumetric (Figure 3), structural characteristics, conventional imaging and histopathological results of the normal and FGR placentas are summarized in Table 1. FGR cases were significantly older, and despite that they had significantly lower placental volume. In addition, they had significantly more marginal cord-insertion location. As expected, FGR had smaller total body (p<0.001) and brain volume percentiles (p=0.1) compared to normal cases. No significant differences were detected at the group level between normal and FGR fetuses in the PBF and ATT values, however, two cases had lower PBF and all cases were significantly older. In the absence of normal values at these late GA (~36 weeks), a further investigation is needed. Radiological findings and histopathological assessment supported abnormal structure of the FGR placentas.
Conclusions
- This study provides normal perfusion values using pCASL at late GA, supporting increased PBF and ATT values with GA.
- This study demonstrates non-invasive comprehensive assessment of the human placental function and structure, and quantitative assessment of fetal development in normal fetuses and in a sample of fetuses diagnosed with FGR.
- Multi-parametric assessment of placental and fetal development may provide biomarkers for the early identification of fetuses with high risk for FGR.
Acknowledgements
This work was supported by the Israel Innovation Authority.References
1. Leitner, Y., Fattal-Valevski, A., Geva, R., Eshel, R., Toledano-Alhadef, H., Rotstein, M., Bassan, H., Radianu, B., Bitchonsky, O., Jaffa, A.J., et al. (2007). Neurodevelopmental outcome of children with intrauterine growth retardation: a longitudinal, 10-year prospective study. J Child Neurol 22, 580-587.
2. Pardi, G., Marconi, A.M., and Cetin, I. (2002). Placental-fetal interrelationship in IUGR fetuses--a review. Placenta 23 Suppl A, S136-141.
3. Harteveld, A.A., Hutter, J., Franklin, S.L., Jackson, L.H., Rutherford, M., Hajnal, J.V., van Osch, M.J.P., Bos, C., and De Vita, E. (2020). Systematic evaluation of velocity-selective arterial spin labeling settings for placental perfusion measurement. Magn Reson Med 84, 1828-1843.
4. Zun, Z., Zaharchuk, G., Andescavage, N.N., Donofrio, M.T., and Limperopoulos, C. (2017). Non-Invasive Placental Perfusion Imaging in Pregnancies Complicated by Fetal Heart Disease Using Velocity-Selective Arterial Spin Labeled MRI. Scientific reports 7, 16126.
5. Shao, X., Liu, D., Martin, T., Chanlaw, T., Devaskar, S.U., Janzen, C., Murphy, A.M., Margolis, D., Sung, K., and Wang, D.J.J. (2018). Measuring human placental blood flow with multidelay 3D GRASE pseudocontinuous arterial spin labeling at 3T. Journal of magnetic resonance imaging : JMRI 47, 1667-1676.
6. Baschat, A.A. (2010). Fetal growth restriction - from observation to intervention. Journal of perinatal medicine 38, 239-246.
7. Gal Dudovitch, D.L.-S., Liat Ben Sira, Elka Miller, Dafna Ben Bashat, Leo Joskowicz. (2020). Deep Learning Automatic Fetal Structures Segmentation in MRI Scans with Few Annotated Datasets. Medical Image Computing and Computer Assisted Intervention – MICCAI 2020, 365-374.
8. Kiserud, T., Piaggio, G., Carroli, G., Widmer, M., Carvalho, J., Neerup Jensen, L., Giordano, D., Cecatti, J.G., Abdel Aleem, H., Talegawkar, S.A., et al. (2017). The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight. PLoS medicine 14, e1002220.
9. Link, D., Braginsky, M.B., Joskowicz, L., Ben Sira, L., Harel, S., Many, A., Tarrasch, R., Malinger, G., Artzi, M., Kapoor, C., et al. (2018). Automatic Measurement of Fetal Brain Development from Magnetic Resonance Imaging: New Reference Data. Fetal diagnosis and therapy 43, 113-122.
10. Shinozuka, N., Okai, T., Kohzuma, S., Mukubo, M., Shih, C.T., Maeda, T., Kuwabara, Y., and Mizuno, M. (1987). Formulas for fetal weight estimation by ultrasound measurements based on neonatal specific gravities and volumes. Am J Obstet Gynecol 157, 1140-1145.
11. Link, D., Many, A., Ben Sira, L., Tarrasch, R., Bak, S., Kidron, D., Gordon, Z., Yagel, S., Harel, S., and Ben Bashat, D. (2020). Placental vascular tree characterization based on ex-vivo MRI with a potential application for placental insufficiency assessment. Placenta 101, 252-260.
12. Konje, J.C., Kaufmann, P.,
Bell, S.C., and Taylor, D.J. (2001). A longitudinal study of quantitative
uterine blood flow with the use of color power angiography in appropriate for
gestational age pregnancies. Am J Obstet Gynecol 185, 608-613.
Figures