3880
Prediction Model for Histological Grade of Endometrioid Adenocarcinoma: Based on Amide Proton Transfer-weighted Imaging and Multimodel DWI1Department of Radiology, Zhengzhou University People’s Hospital & Henan Provincial People’s Hospital, Zhengzhou, China, 2Department of Radiology, Henan University People’s Hospital & Henan Provincial People’s Hospital, Zhengzhou, China, 3Department of MRI, The First Affiliated Hospital of Xinxiang Medical University,, Weihui, China, 4GE Healthcare, MR Research China, BeiJing, China
Synopsis
Our results showed that both multimodel DWI and APTWI can estimate the histological grade and Ki-67 index of endometrial adenocarcinoma (EA), and the combination of a high MTRasym(3.5 ppm) and low D may be an effective imaging marker for predicting the grade of EA.
Introduction
Endometrial adenocarcinoma (EA) is one of the most common malignancies of the female reproductive system[1]. Conventional MRI sequences reflect only the morphological characteristics of lesions, making it challenging to pre-evaluate the microscopic information of EA such as histological grade and Ki-67 index. Multimodel diffusion-weighted imaging (DWI), including monoexponential, biexponential, and stretched exponential models, is sensitive to the water molecular diffusion, microcirculation perfusion, and heterogeneity in biological tissue[2, 3, 4]. Amide transfer- weighted imaging (APTWI) is a molecular imaging technology that can achieve noninvasive quantitative assessment of mobile proteins and polypeptides concentrations in tissues without the use of contrast agents[5]. At present, only a few small sample studies have reported the application of a monoexponential DWI model and APTWI in the histological grade of EA[2, 6]. This study aimed to investigate whether parameters derived from APTWI and multimodel DWI can establish a prediction model for the histological grade of EA, and to analyze correlation between each parameter and the Ki-67 index.Material and Methods
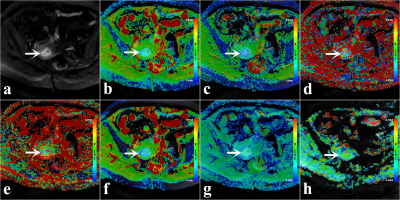
A total of 90 enrolled subjects were classified into low-grade (grade 1, 2) and high-grade (grade 3) groups. A 3.0-T MR scanner (Discovery MR750, GE Healthcare) with a 16-channel phased-array body coil was performed. First, axial T1-weighted imaging (T1WI) and T2-weighted imaging (T2WI) were performed. Next, regarding the above images, the slices where a tumor appeared to be present were selected as the scan sections for multimodel DWI and APTWI. The monoexponential model was performed by using two b values (0, 1000 s/mm2). The biexponential and stretched models were performed by using ten b values (0, 25, 50, 100, 150, 200, 400, 600, 800, and 1000 s/mm2). APTWI was conducted by using a saturation pulse (Tsat) with a duration of 0.5 s and a saturation power level of 2.0 μT. A total of 52 frequencies, including 49 offsets ranging from -600 to +600 Hz with an interval of 25 Hz and a frequency 5000 Hz (3 times) far from the resonant frequency, were used for the APTWI and z-spectrum scans for signal normalization. The water saturation shift reference (WASSR) was applied for B0 correction. The ROIs, excluding areas with large vessels, hemorrhagic, calcified, cystic, and necrotic, were drawn along the edge at the maximum cross-section of the tumor.MedCalc 15.0 and SPSS 23.0 were employed for statistical analyses. The independent sample t- test and Mann-Whitney U test were applied for between-group comparison. The receiver operating characteristic (ROC) curve and Delong test were performed to evaluate and compare the diagnostic performance of each parameter. The logistic regression analyses were used to derive a prediction model. The Pearson correlation were employed to describe the correlation of each parameter with the Ki-67 index, respectively. Results with P < 0.05 were considered to be significant.
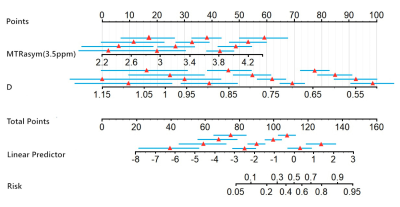
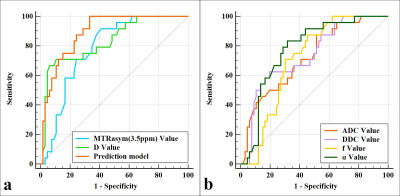
Results
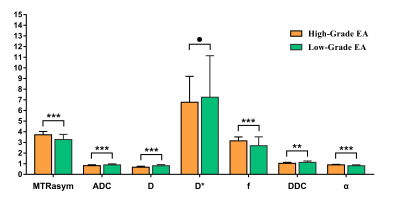
The MTRasym (3.5 ppm), f, and α were higher and ADC, D, and DDC were lower in the high-grade group than in the low-grade group. The difference in D* between the two groups was not significant . Among age, tumor size, FIGO stage and related parameters, only MTRasym(3.5 ppm) and D were independent predictors for the histological grade of EA. The formula of the prediction model was as follows: Logit (P) = - 2.507 + 0.920 × MTRasym(3.5 ppm) - 1.886 × D. For the diagnosis of high- and low-grade EA, AUC (prediction model) > AUC (D) > AUC (MTRasym (3.5 ppm)) > AUC (α) > AUC (DDC) > AUC (ADC) > AUC (f), where the differences between AUC (prediction model) and AUC (MTRasym(3.5 ppm)), AUC (α), AUC (DDC), AUC (ADC), and AUC (f) were significant (Z = 2.512, 2.433, 3.052, 3.818, and 3.229, respectively). Regarding the comparison between various parameters, only the difference between AUC (D) and AUC (ADC) was significant (Z = 2.499, P = 0.012) .The correlation between each parameter and the Ki-67 index was as follows: MTRasym (3.5 ppm), r = 0.395; α, r = 0.367; D, r = -0.529; DDC, r = -0.515; ADC, r = -0.427; and f, r = 0.355 (Figure1-4).Discussion
Compared with low-grade EA, high-grade EA have an increase in cellular density, nuclear atypia, microvessel density (MVD), and microscopic necrosis[6, 7, 8], which not only can limit the diffusion velocity of water molecules, also can increase the contents of mobile proteins and polypeptides, capillary microcirculation perfusion, and intravoxel diffusion heterogeneity, leading to changes in signal intensity (SI) of APTWI and multimodel DWI. The reasons for no difference in D* value between high- and low- grade EA may be as follows: the opposite influence of the capillary segment length and average blood velocity, poor measurement reproducibility, low signal-to-noise ratio (SNR), and tumor heterogeneity[9]. One possible explanation for the correlations between different parameters and the Ki-67 index is that a high Ki-67 index correlates with increased cell proliferation[10], which tends to possess higher cellular density, higher MVD and more complex tissue structure, leading to changes in various parameter values.Conclusion
Both multimodel DWI and APTWI can be used to estimate the histological grade and Ki-67 index of EA, and the combination of a high MTRasym(3.5 ppm) and low D may be an effective imaging marker for predicting the grade of EA.Acknowledgements
The National Key R&D Program of China (2017YFE0103600), the Henan Medical Science and Technology Research Program (2018020357 and 2018020367), the National Natural Science Foundation of China (81720108021 and 31470047), and Zhongyuan Thousand Talents Plan Project - Basic Research Leader Talent (ZYQR201810117).References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69(1):7-34.
2. Nougaret S, Reinhold C, Alsharif SS, et al. Endometrial Cancer: Combined MR Volumetry and Diffusion-weighted Imaging for Assessment of Myometrial and Lymphovascular Invasion and Tumor Grade. Radiology. 2015;276(3):797-808.
3. Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2): 497-505.
4. Bennett KM, Schmainda KM, Bennett RT, Rowe DB, Lu H, Hyde JS. Characterization of continuously distributed cortical water diffusion rates with a stretched-exponential model. Magn Reson Med. 2003;50(4):727-734.
5. Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9(8):1085-1090.
6. Takayama Y, Nishie A, Togao O, et al. Amide Proton Transfer MR Imaging of Endometrioid Endometrial Adenocarcinoma: Association with Histologic Grade. Radiology. 2018; 286(3): 90-917.
7. Dobrzycka B, Mackowiak-Matejczyk B, Kinalski M, et al. Pretreatment serum levels of bFGF and VEGF and its clinical significance in endometrial carcinoma. Gynecol Oncol. 2013;128(3): 454-460.
8. Fukunaga T, Fujii S, Inoue C, et al. Accuracy of semiquantitative dynamic contrast-enhanced MRI for differentiating type II from type I endometrial carcinoma. J Magn Reson Imaging. 2015; 41(6):1662-1668.
9. Liu C, Wang K, Chan Q, et al. Intravoxel incoherent motion MR imaging for breast lesions: comparison and correlation with pharmacokinetic evaluation from dynamic contrast-enhanced MR imaging. Eur Radiol. 2016;26(11):3888-3898.
10. Zhang J, Chen X, Chen D, Wang Z, Li S, Zhu W. Grading and proliferation assessment of diffuse astrocytic tumors with monoexponential, biexponential, and stretched-exponential diffusion-weighted imaging and diffusion kurtosis imaging. Eur J Radiol. 2018;109:188-195.
Figures