3870
A novel method for temperature mapping in fatty tissues based on Z-Spectrum MRI with binomial pulse
Alessandro Scotti1,2, Jin Gao3, Weiguo Li3, Mehran Shaghaghi1, Fred Damen1, and Kejia Cai1,2
1Radiology, University of Illinois at Chicago, Chicago, IL, United States, 2Bioengineering, University of Illinois at Chicago, Chicago, IL, United States, 3Research Resource Center, University of Illinois at Chicago, Chicago, IL, United States
1Radiology, University of Illinois at Chicago, Chicago, IL, United States, 2Bioengineering, University of Illinois at Chicago, Chicago, IL, United States, 3Research Resource Center, University of Illinois at Chicago, Chicago, IL, United States
Synopsis
The measurement of temperature in fatty tissues remains a challenge, since the presence of fat protons modifies the phase difference and therefore introduces errors in the quantification from proton resonance frequency shift. By using Z-spectrum MRI, combined with a Jump-Return binomial pulse preparation, we create a sinusoidal variation of the signal as a function of the resonance frequency. The magnitude-based readout eliminates the risk of phase-related artifacts and allows to measure the subtle shifts of water resonance and therefore the temperature, even in fatty tissues. We tested the sequence in mice and cream phantom.
Introduction
Temperature mapping is a fundamental need in biological research and noninvasive methods to reliably assess the variation in temperature due to pathology progression or treatment are highly wanted. In the MRI realm, the most widely used technique for temperature measurement is Proton Resonance Frequency (PRF), which relies on the change of water chemical shift triggered by a temperature-dependent modulation of the hydrogen’s electronic shielding1. The measurement is often performed by tracking the change in the MR phase with respect to a reference temperature state. However, the measurement of temperature in fatty tissues remains a challenge, since the presence of fat protons modifies the phase difference and therefore introduces errors in the quantification2. Here we propose a new technique based on the combination of Z-spectrum MRI and a binomial pulse preparation, the Jump-Return (JR) sequence3, to measure the subtle shifts of water resonance and therefore the temperature.Methods
The sequence consisted on a Z-Spectrum MRI sequence, with a preparation segment applied at a range of different offsets, immediately followed by a FSE readout. The preparation entailed a binomial pulse with structure 1-1, where the magnetization is flipped by a first 90° pulse, allowed to evolve during the delay τ, and then flipped back to the yz-plane by a second pulse with opposite phase. By this mechanism, on-resonance protons do not evolve in the rotating frame and their magnetization is fully flipped back onto the z-axis. Close off-resonance spins will dephase during τ according to their chemical shift and will have less magnetization available at readout. At larger chemical shifts, the magnetization is rephased, resulting in a sinusoidal behaviour of the signal amplitude as a function of chemical shift, with null/maxima values determined by the delay-resonance product (f * τ). When temperature changes, the water resonance f shifts and with it the curve. By fitting the curve with a sin function of (f * τ), it is possible to keep track of the shift and therefore of the change in temperature (Fig.1). As a proof of principle, experiments were first carried out on a phantom containing phosphate buffer solution (PBS) at 9.4 T preclinical scanner. Temperature in the phantom was increased by regulating the warm air flow into the scanner bore. An MRI- compatible physiological monitoring system with the sensor inserted in the phantom monitored the thermal changes in real time. The JR sequence consisted on a preparatory module of two sinc-shaped pulses 0.3 ms long, separated by a 3 ms long delay. Both pulses produced a 90° flip angle and had opposite phase. Following the preparation, a FSE readout with 2 segments and a 64x32 matrix collected the signal. The temperature change was then computed as ΔT= Δf/α, where Δf is the frequency shift and α=0.01 °C/ppm in most aqueous tissues. Interleaved to the JR sequences, also multi echo gradient echo (min TE=0.85ms, 3 echoes) sequences were acquired, and temperature variation derived from the phase differences. The protocol was then tested in vivo on the inguinal region of healthy mice exposed to temperature variation. Finally, the sequence was also tested on a phantom containing heavy whipped cream (fat content 25%). For the study of fatty tissues, the signal was fitted to the sum of two sin functions with a relative phase offset of 3.5 ppm.Results
The signal modulation from PBS as a function of frequency offset is shown in Fig.1. As the temperature in the phantom increases, the water resonance shifts downfield and so does the curve. The fit by the sinusoidal function was accurate (R2>0.98) everywhere, independently from the initial B0 inhomogeneity. Fig.2 shows the water frequency shift in all tissues during the in vivo experiment. The shift in the tissue is proportional to the outside temperature variation. Interestingly, the shift in the fat depots is of opposite polarity than in the muscular tissue. Finally, also the temperature variation measured by the JR in the cream phantom was found consistent with the sensor measurement (Fig.3), whereas the phase differences from GRE, as expected, produced wild variability when the fat and water protons were not in-phase.Conclusion
Here we introduced a novel combination of the simple JR sequence and of the Z-Spectrum MRI platform in order to measure temperature-induced frequency shifts in fatty tissues. The technique is noninvasive, fast, high resolution, and highly sensitive (<1°C). By being magnitude-based, it is inherently insensitive to phase errors and can therefore overcome the long-standing hurdle of measuring temperature in tissues with mixed compositions of fat and water.Acknowledgements
No acknowledgement found.References
1. V Rieke & KB Pauly, JMRI, 2008.
2. Quesson, JMRI, 2000. 3PJ Hore, JMR, 1983.
Figures
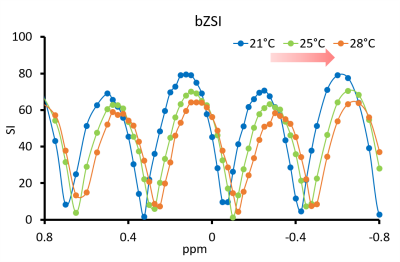
Signal from PBS phantom experiment. The signal in proximity of the water resonance varies as a sin function of the chemical shift. When temperature rises, the curve shifts downfield.
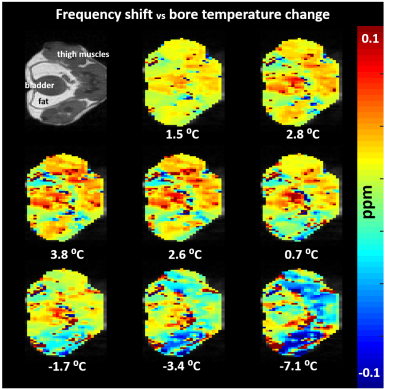
In vivo maps of frequency shift. When the temperature in the scanner bore is changed, the resonance frequency shifts in the tissue, proportionally to the temperature variation.
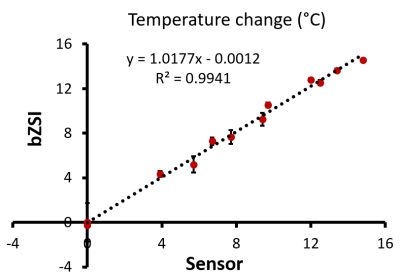
Temperature measurement in cream. The cream phantom serves as an example of mixed water and fat tissue. The temperature measured by the binomial Z-Spectrum MRI correlates well with the ground truth measurement from the sensor inserted in the phantom.