3855
Water/fat separation using multiple decoder U-Net architecture (MDWF-Net) for accurate R2* measurements1Biomedical Imaging Center, Pontificia Universidad Catolica de Chile, Santiago, Chile, 2ANID – Millennium Science Initiative Program – Millennium Nucleus for Cardiovascular Magnetic Resonance, Santiago, Chile, 3Institute for Biological and Medical Engineering, Pontificia Universidad Catolica de Chile, Santiago, Chile, 4Department of Electrical Engineering, Pontificia Universidad Catolica de Chile, Santiago, Chile, 5Radiology Department, School of Medicine, Pontificia Universidad Catolica de Chile, Santiago, Chile, 6Institute for Mathematical & Computational Engineering, Pontificia Universidad Catolica de Chile, Santiago, Chile, 7ANID – Millennium Science Initiative Program – Millennium Nucleus for Discovery of Structure in Complex Data, Santiago, Chile
Synopsis
Proton density fat fraction (PDFF) and $$$R_2^*$$$, key biomarkers associated to liver disease, can be obtained by solving the water/fat separation problem. Convolutional Neural Networks (CNN) have been proposed for solving this problem. However, proposed solutions have not achieved accurate $$$R_2^*$$$ mapping. We introduce MDWF-Net, a CNN model for computing high quality water-fat, $$$R_2^*$$$ and field mapping from abdominal acquisitions. The results were evaluated considering error and structural similarity and compared against a U-Net. We also evaluated ROIs in the liver for PDFF and $$$R_2^*$$$. The proposed MDWF-Net overperforms the original U-Net, especially for $$$R_2^*$$$ maps, even with fewer echoes.
Introduction
Proton density fat fraction (PDFF) and $$$R_2^*$$$ signal decay are important biomarkers associated to non-alcoholic fatty liver disease and iron overload1. Both are measured by solving a water/fat separation problem with field map ($$$\Delta f$$$) corrections, from gradient echo multi-echo acquisitions2. Recently, deep learning methods have been proposed for water/fat separation problem3–6. Most of them are based on convolutional neural networks (CNN) that used a U-Net architecture3,4. This architecture allows for getting water/fat images at a minimal computational cost and high accuracy, after a computationally demanding training process. However, these approaches tend to generate blurred and underestimated results for $$$R_2^*$$$ and field maps, and thus, the solutions are useful for accurate PDFF calculations only.We propose a CNN with a novel architecture that enables a highly accurate solution of the water/fat separation problem and, remarkably, accurate, non-blurred and artifact-free $$$R_2^*$$$ mapping. We trained our model with abdominal multi-slice acquisitions and compared the results against the U-Net model.
Methods
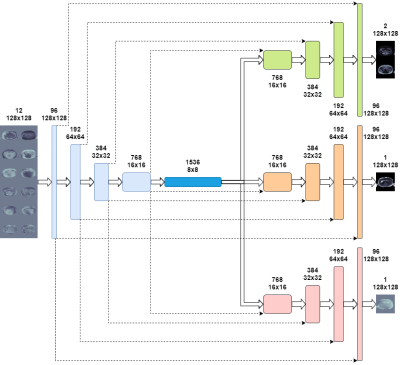
We introduce Multiple Decoded Water/Fat Net (MDWF-Net), a CNN architecture based on U-Net with multiple decoders for water/fat, $$$R_2^*$$$ and $$$\Delta f$$$ (Figure 1). Change for this improvement allows for parameter disentangling, which increase the model’s capabilities to describe the particular behavior of each map.This study used 2D multi-slice axial abdomen images of 101 volunteers, acquired with a gradient echo multi-echo sequence with six echo times (TE1/$$$\Delta$$$TE/TR = 1.3/2.1/30 ms) at 1.5T scanner (Achieva, Philips Healthcare). The final database included 2237 complex images that were rescaled to 128x128 pixels by subsampling in the K-space. The water/fat separation references were obtained with Graph Cut method2. From the overall complex images, 1790 were used for training, 223 for validation and 224 for testing. The loss function was the mean absolute error of the outputs (water, fat, $$$R_2^*$$$, $$$\Delta f$$$). The training setting was as follows: Adam optimizer ($$$\beta_1=0.9$$$, $$$\beta_2=0.999$$$), cosine learning rate decay starting at 0.0005, 120 epochs, batch size of 32. Data augmentation was performed, including vertical and horizontal reflections and vertical translations in the range of $$$\pm 20$$$ pixels. We trained MDWF-Net considering 3,4,5 and 6 echoes, prospectively. All networks were trained using a Tesla T4 GPU.
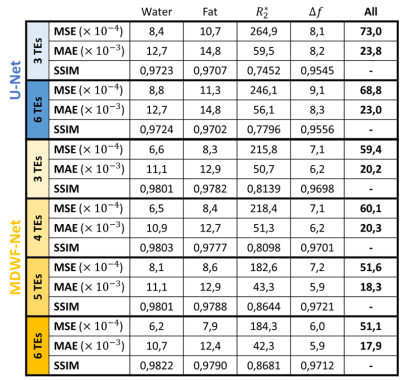
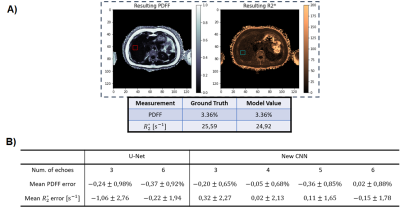
The performance of MDWF-Net was evaluated considering the mean square and absolute errors (MSE, MAE), and the structural similarity index measure (SSIM)7 of the resulting maps with respect to reference results. For comparison purposes, a U-net was trained with the same training setting than MDWF-Net, considering 3 and 6 echoes (Table 1). For an evaluation more compatible with clinical practice, we also considered the error of PDFF and $$$R_2^*$$$ at regions of interest (ROIs), considering one ROI per image and discarding slices with negligible hepatic tissue.
Results
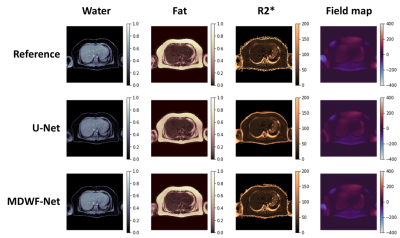
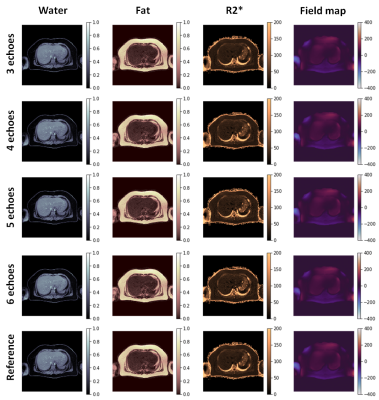
MDWF-Net outperforms the original U-Net, especially for $$$R_2^*$$$ (Figure 2), even with a reduced number of echoes (Figure 3). Compared to the Graph Cut references, MSE, MAE and SSIM showed better performance for the proposed model, with a remarkable improvement in $$$R_2^*$$$ (Table 1). SSIM of $$$R_2^*$$$ highlights the major improvement in deblurring and artifact reduction produced by MDWF-Net compared to U-Net, even with 3 echoes (Table 1).PDFF and $$$R_2^*$$$ values at liver ROIs showed lower mean absolute error for our proposed model. Considering the different number of echoes, MDWF-Net achieved lower errors and standard deviations than U-Net for all cases, especially for $$$R_2^*$$$ (Figure 4). Results showed that similar quality $$$R_2^*$$$ mapping can be achieved with 5 echoes.
Discussion
MSE, MAE and SSIM showed that MDWF-Net with 3 echoes overperformed U-Net with 6 echoes (Table 1). They also showed that MDWF-Net was capable of computing high quality water and fat images even with 3 echoes, but $$$R_2^*$$$ and $$$\Delta f$$$ maps were more affected by the reduction of echoes. Nevertheless, a similar accuracy in $$$R_2^*$$$ could be achieved with 5 echoes. In the case of field map, even a reduction of one echo affects the performance. Despite these results, $$$R_2^*$$$ and $$$\Delta f$$$ maps were qualitatively acceptable even with fewer echoes, while water and fat estimations remained accurate (Figure 3).Finally, the mean and standard deviation of the absolute errors in ROIs corroborated the high precision of $$$R_2^*$$$ mapping, particularly in the hepatic tissue (Figure 4b). The results also showed that the PDFF precision of the MDWF-Net overperformed U-Net, which is noticeable with the lower standard deviations (Figure 4b). PDFF error in ROIs showed that MDWF-Net performance remained unaffected with fewer echoes.
Conclusion
We proposed MDWF-Net, a U-Net based architecture with multiple decoders, to solve the water/fat separation problem. MDWF-Net generates more accurate and precise estimations of the PDFF and $$$R_2^*$$$ at the hepatic tissue, compared with the original U-Net.Even with considerably less echoes, MDWF-Net still achieved high quality quantifications, especially for $$$R_2^*$$$. This could be a considerable advantage for shortening the gradient echo acquisitions required to solve the water/fat separation problem.
In future work, we will incorporate simulated data to the training of the model to improve the quality of the training process, increasing the number of data samples and the robustness of the resulting CNN. The inclusion of simulated data will also allow to have completely accurate ground-truth labels and to test with other echo times.
Acknowledgements
This work was funded by ANID – Millennium Science Initiative Program – NCN17_129 and PIA/Anillo ACT 192064. C.A. was partially funded by CONICYT FONDECYT Postdoctorado 2019 #3190763, CONICYT PCI REDES 180090, and ANID – Millennium Science Initiative Program – NCN17_129. C.SL. was partially funded by ANID – Millennium Science Initiative Program – NCN17_059.References
1. Starekova, J. & Reeder, S. B. Liver fat quantification: where do we stand? Abdom. Radiol. 45, 3386–3399 (2020).
2. Hernando, D., Kellman, P., Haldar, J. P. & Liang, Z. P. Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magn. Reson. Med. 63, 79–90 (2010).
3. Andersson, J., Ahlström, H. & Kullberg, J. Separation of water and fat signal in whole-body gradient echo scans using convolutional neural networks. Magn. Reson. Med. 82, 1177–1186 (2019).
4. Goldfarb, J. W., Craft, J. & Cao, J. J. Water–fat separation and parameter mapping in cardiac MRI via deep learning with a convolutional neural network. J. Magn. Reson. Imaging 50, 655–665 (2019).
5. Cho, J. J. & Park, H. W. Robust water–fat separation for multi-echo gradient-recalled echo sequence using convolutional neural network. Magn. Reson. Med. 82, 476–484 (2019).
6. Liu, K. et al. Robust water–fat separation based on deep learning model exploring multi-echo nature of mGRE. Magn. Reson. Med. 1–14 (2020) doi:10.1002/mrm.28586.
7. Wang, Z., Bovik, A. C., Sheikh, H. R. & Simoncelli, E. P. Image quality assessment: From error visibility to structural similarity. IEEE Trans. Image Process. 13, 600–612 (2004).
Figures