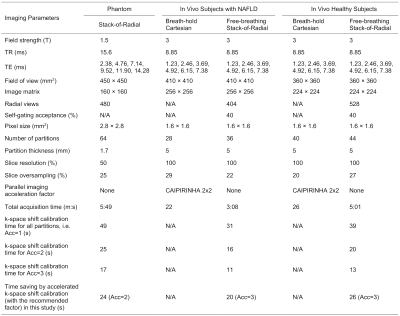
3845
Improved Efficiency of Free-Breathing Stack-of-Radial MRI for Liver Fat and R2* Quantification Using Accelerated k-space Shift Calibration1MR R&D Collaborations, Siemens Medical Solutions USA, Inc., Los Angeles, CA, United States, 2Radiological Sciences, University of California Los Angeles, Los Angeles, CA, United States, 3Physics and Biology in Medicine Interdepartmental Program, University of California Los Angeles, Los Angeles, CA, United States, 4MR Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany, 5MR R&D Collaborations, Siemens Medical Solutions USA, Inc., Cary, NC, United States, 6MR R&D Collaborations, Siemens Medical Solutions USA, Inc., Austin, TX, United States
Synopsis
For liver proton density fat fraction (PDFF) and R2* quantification, free-breathing three-dimensional stack-of-radial MRI may perform a calibration scan for k-space shift correction. This study proposes an accelerated k-space shift calibration method. The proposed method was validated in a PDFF/R2* phantom with vendor-provided ground truth. Preliminary in vivo results demonstrated good agreement in the liver PDFF/R2* compared to the reference breath-hold Cartesian MRI. This proposed method enabled a 3-fold reduction in calibration time (20 seconds or more) for the in vivo protocols in this study. It may allow more efficient free-breathing PDFF/R2* mapping in patient populations with breath-hold difficulties.
INTRODUCTION
Three-dimensional (3D) multi-echo stack-of-radial MRI can be used for free-breathing acquisitions for liver proton density fat fraction (PDFF) and R2* quantification with a calibration scan for k-space shift correction and self-gating for respiratory motion compensation.1-9 While self-gating uses imaging data and has no scan time penalty, k-space shift calibration scans typically consume 30 seconds or more. For reference, the imaging acquisition time is usually 2-5 minutes.5-9 The purposes of this study were to develop an accelerated k-space shift calibration method, and to validate it in a phantom and subjects versus a reference breath-hold 3D Cartesian method.METHODS
Accelerated Calibration Scan for k-space Shift CorrectionThe considered calibration scan acquires data for all 3D partitions and calculates k-space shift data as a function of the partition and coil channel indices.3-5 In this work, an acceleration factor Acc was used to acquire data for only one partition in every Acc partitions. The missing k-space shift data were interpolated by a cubic spline using the data of the neighboring acquired partitions.
Pulse Sequences and Image Reconstruction
A multi-echo GRE stack-of-radial prototype sequence7,8 with k-space shift calibration was employed.3-5 Raw data were saved for retrospective reconstruction with different Acc values. For in vivo acquisitions, self-gating was applied to accept data acquired near end-expiration with a 40% acceptance rate,10-11 and a breath-hold multi-echo 3D Cartesian GRE prototype sequence12 was utilized for reference. Both sequences applied the same multi-step adaptive fitting algorithm for simultaneous PDFF and R2* quantification.14 For the phantom, mono-exponential fitting was used to calculate R2*.
Phantom Experiment and Data Analysis
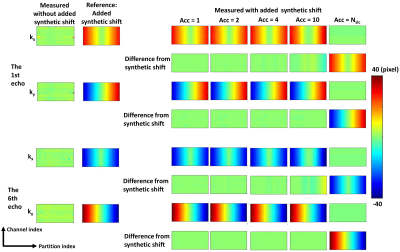
From a previous study,13 stack-of-radial data of a phantom (Calimetrix, Madison, Wisconsin, USA) scanned at 1.5T (MAGNETOM AvantoFit, Siemens Healthcare, Erlangen, Germany) was processed. The phantom had 7 vials with vendor-provided R2* values of 28.8, 50.3, 92.8, 146.9, 275.8, 573.4 and 946.8 s-1 (the last two vials were excluded in the analysis due to high iron), and 8 vials with vendor-provided PDFF values of 0, 2.7, 4.7, 7.6, 14.4, 30.2, 50.6 and 100%. Imaging parameters are listed in Table 1. To exaggerate the influence of k-space shift for better illustration, for the phantom data only, synthetic k-space shift values were added to the original stack-of-radial k-space data without any k-space shift correction before reconstruction (Figure 1).
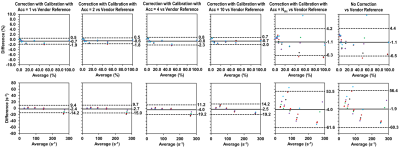
Images were reconstructed without k-space shift correction, and with correction from calibration data acquired with acceleration factors Acc = 1 (acquiring every partition), 2, 4, 10, and Nslc (number of partitions; equivalent to acquiring the center partition only) in the partition direction. PDFF and R2* values inside vials on 6 neighboring partitions were measured and compared to vendor-provided values using Bland-Altman Analysis.
In Vivo Experiment and Data Processing
This study was HIPAA-compliant and IRB-approved. After obtaining written informed consent, data from 3 healthy subjects (2 females, 23.7±3.5 yrs, body mass index 24.3±3.6 kg/m2) and 3 subjects with non-alcoholic fatty liver disease (NAFLD) (2 females, 62.7±7.2 yrs, body mass index 30.2±5.5 kg/m2) were acquired at 3T (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany). Data of the 3 subjects with NAFLD were included from a previous study;9 therefore, Bayesian statistical analysis was performed to eliminate multiple comparison concerns. Imaging parameters are listed in Table 1.
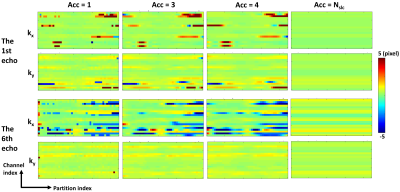
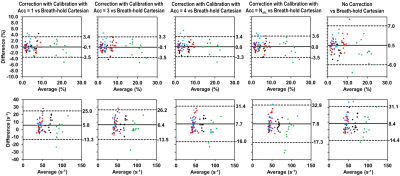
Images were reconstructed without k-space shift correction, and with correction with acceleration factors Acc = 1, 3, 4 and Nslc. Twelve region-of-interests (ROIs) were manually placed on four slices as described previously.7 PDFF and R2* values inside these ROIs were measured and compared using Bland-Altman Analysis.
RESULTS
Time savings by accelerated k-space shift calibration scans for the protocols used in this study were listed in Table 1.The reference synthetic k-space shift map and the measured k-space shift maps using different Acc factors for the phantom are shown in Figure 1. In the Bland-Altman plots of PDFF and R2*, mean differences (MD) and limits of agreement (LoA) measured without correction and with Acc=Nslc calibration were the largest, while those measured with Acc=1 calibration had the smallest MD and LoA, similarly for Acc=2 (Figure 2). Generally, the larger Acc was, the larger MD and LoA were.
The measured k-space shift map of one representative subject with NAFLD is shown in Figure 3, where maps measured with Acc=3 were similar to those measured with Acc=1. In Figure 4, measurements without correction exhibited the largest MD and LoA for both PDFF and R2*. Measurements with Acc= Nslc calibration also exhibits similar MD and LoA for R2*, but not for PDFF. Measurements of both PDFF and R2* with correction from Acc=3 calibration had small MD and LoA, similar to measurements with Acc=1. The trend that the larger Acc was, the larger MD and LoA were was also observed.
DISCUSSION AND CONCLUSION
An acceleration method was developed for k-space shift calibrations in 3D stack-of-radial MRI and evaluated in a phantom and subjects. This study demonstrated agreement of liver PDFF and R2* compared to the reference breath-hold Cartesian results. A 3-fold reduction in calibration time (20 seconds or more) was achieved for the in vivo protocols in this study. This proposed method may allow more efficient free-breathing stack-of-radial MRI PDFF/R2* mapping in patient populations with difficulties holding breath.Acknowledgements
We would like to thank Shu-Fu Shih for verifying the in vivo data. This study was supported in part by Siemens Medical Solutions USA, Inc and the Department of Radiological Sciences at UCLA.References
1. Chandarana H, Block TK, Rosenkrantz AB, Lim RP, Kim D, Mossa DJ, Babb JS, Kiefer B, Lee VS. Free-breathing radial 3D fat-suppressed T1-weighted gradient echo sequence: a viable alternative for contrast-enhanced liver imaging in patients unable to suspend respiration. Invest Radiol 2011;46:648-653.
2. Chandarana H, Feng L, Block TK, Rosenkrantz AB, Lim RP, Babb JS, Sodickson DK, Otazo R. Free-breathing contrast-enhanced multiphase MRI of the liver using a combination of compressed sensing, parallel imaging, and golden-angle radial sampling. Invest Radiol 2013;48:10-16.
3. Reeder SB, Atalar E, Faranesh AZ, McVeigh ER. Referenceless interleaved echo-planar imaging. Magn Reson Med. 1999;41:87-94.
4. Peters DC, Derbyshire JA, McVeigh ER. Centering the projection reconstruction trajectory: reducing gradient delay errors. Magn Reson Med 2003;50:1-6.
5. Armstrong T, Dregely I, Stemmer A, Han F, Natsuaki Y, Sung K, Wu HH. Free-breathing liver fat quantification using a multiecho 3D stack-of-radial technique. Magn Reson Med 2018;79:370-382.
6. Armstrong T, Ly KV, Murthy S, Ghahremani S, Kim GHJ, Calkins KL, Wu HH. Free-breathing quantification of hepatic fat in healthy children and children with nonalcoholic fatty liver disease using a multi-echo 3-D stack-of-radial MRI technique. Pediatr Radiol 2018;48:941-953.
7. Zhong X, Armstrong T, Nickel MD, et al. Effect of respiratory motion on free-breathing 3D stack-of-radial liver R2* relaxometry and improved quantification accuracy using self-gating. Magn Reson Med 2020;83:1964-1978.
8. Zhong X, Hu HH, Armstrong T, Li X, Lee YH, Tsao TC, Nickel MD, Kannengiesser SAR, Dale BM, Deshpande V, Kiefer B, Wu HH. Free-Breathing Volumetric Liver R2* and Proton Density Fat Fraction Quantification in Pediatric Patients Using Stack-of-Radial MRI With Self-Gating Motion Compensation. J Magn Reson Imaging 2020;doi: 10.1002/jmri.27205.
9. Armstrong T, Zhong X, Hu HH. Free-breathing liver fat quantification in adults with NAFLD using a 3D stack-of-radial MRI technique. Proc. Intl. Soc. Mag. Reson. Med. 27, 2019. p1028.
10. Grimm R, Block KT, Hutter J, Forman C, Hintze C, Kiefer B, Hornegger J. Self-gating reconstructions of motion and perfusion for free-breathing T1-weighted DCE-MRI of the thorax using 3D stack-of-stars GRE imaging. Proc. Intl. Soc. Mag. Reson. Med. 20, 2012. p598.
11. Grimm R, Bauer S, Kiefer B, Hornegger J, Block T. Optimal channel selection for respiratory self-gating signals. Proc. Intl. Soc. Mag. Reson. Med. 21, 2013. p3749.
12. Zhong X, Nickel MD, Kannengiesser SAR, Dale BM, Kiefer B, Bashir MR. Liver fat quantification using a multi-step adaptive fitting approach with multi-echo GRE imaging. Magn Reson Med 2014;72:1353-1365.
13. Gao C, Hu P, Dale B, Nickel MD, Kannengiesser SAR, Kiefer B, Deshpande V, Zhong X. A stability study of breath-hold and free-breathing liver fat and R2* quantification at 1.5T and 3T. Proc. Intl. Soc. Mag. Reson. Med. 28, 2020. p3165.
14. Breuer FA, Blaimer M, Mueller MF, Seiberlich N, Heidemann RM, Griswold MA, Jakob PM. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magn Reson Med 2006;55:549-556.
Figures