3839
Healthy human aging and sex dimorphism in bone-muscle cross talk: a 1H MRS investigation in bone marrow and muscle of legs.1Physics Dpt Sapienza, CNR ISC, Rome, Italy, 2Santa Lucia foundation, Rome, Italy, 3CNR ISC, Rome, Italy, 4Museo Storico della Fisica e Centro Studi e Ricerche Enrico Fermi, Rome, Italy, 5Physics Dpt., Sapienza University, Rome, Italy, 6Tor Vergata University, PTV, Rome, Italy
Synopsis
Aim of this study was to identify relevant changes in the metabolic profile of bone-marrow and muscle during healthy aging in both men and women. Towards this goal, single-voxel MRS with STEAM at TE=6ms and T2 evaluation of each resonance was performed. Bone-marrow fatty-acids quantification show a clear sex dimorphism with normal aging while no significant differences were found in the muscle metabolites of female and male subjects. On the other hand, metabolite-T2 values were different in male and female resonances and significant T2 differences among 36+, 36-52 and 52+ group, finding higher T2 in older group were observed.
Introduction
Aging is associated with both losses of bone mineral density (BMD) and increased fracture risk (i.e., osteoporosis) and sarcopenia (i.e., loss of muscle strength and mass). There is now growing evidence that the mechanical/endocrine relationship and the bidirectional communication between muscle and bone can determine the impact of aging and the severity of the decline in muscle size and bone fragility. Moreover, there is increasing evidence that age-associated changes in body composition may severely affect fat tissue and adipose1,2 In this view, 1H-MRS is an excellent tool for investigating muscle3 and has proved to be a useful tool for investigating bone quality through the quantification of bone marrow fatty acids4. The aim of this study was to identify relevant changes in the metabolic profile of bone-marrow and muscle during healthy aging in both men and women. A better understanding of the bone-muscle connection, and gender-associated differences, via the alterations of selected fatty-acids, may pave the way for novel medical interventions in musculoskeletal diseases such as sarco-osteoporosis.Methods
Thirty-two Caucasian subjects (age range 18-82 years, 18 female, 14 male) were recruited to investigate tibial bone-marrow and soleus/gastrocnemius muscle. Subjects were excluded in the case of clinical evidence for metabolic bone disease or metastasis, previous history of irradiation, and current use of steroids or hormone replacement therapy. From each volunteer, clinical data (age, sex, body-mass-index, BMD) were collected. The study was approved by the Local Ethics Committee. Written informed consent was obtained from each subject before the MRS-examination. Experiments: Single-voxel-MRS was performed by STEAM sequence with TE=6ms. Specifically, 20x20x30mm3 voxel-size, acquired with VAPOR sequence, NS=48, TR=5s, TE=6,35,135ms were used to obtain muscle-spectra and to evaluate the T2 of each resonance. A 8x8x30mm3 voxel-size, NS=32, TR=5s, TE=6,35,60ms were used to obtain bone-marrow-spectra and to evaluate T2 of each resonance. Processing and statistical analysis: Row data of spectra acquired from each subject were analyzed using LcModel software5 (SPTYPE 6). Zero and first-order phase-corrections were performed. Nine proton-resonances were quantified in bone-marrow spectra: CH3 (labeled L09, at 0.90ppm), bulk-CH2 (L13 at 1.3ppm), CH2α- (L23 at 2.3ppm) and β- (L16 at 1.59ppm) to the carbonyl, allylic-CH2 α to a double-bound (L21 at 2.03ppm), water (W at 4.7ppm), glycerol CH2, glycerol CH (L41+L43 at 4.10-4.35ppm) and olefinic double-bond protons (L52+L53 at 5.20ppm). Moreover, from muscle-spectra we quantified: Intramyocellular lipids (IMCL at 1.3ppm), extramyocellular lipids (EMCL at 1.5ppm), creatine (Cr at 3.0ppm), choline (Cho at 3.2ppm), taurine, and other fatty-acids resonances. Each bone-marrow and muscle resonance was computed as mean±SD. Data with a %SD equal to or higher than 10% were discarded.T2 of each resonance was calculated. Each resonance area obtained at TE=6ms was corrected for its T2 value. Homogeneity of variances between groups (subjects with age less than 36y, 36-, from 36 to 52y, 36-52, and higher than 52y, 52+, female and male) was tested by Levene’s test. Pairwise comparisons between groups were made using a Welch ANOVA. Games-Howell corrections were performed to correct for multiple testing. Pearson correlation was studied between fatty acids/ metabolites and age.Results
Examples of tibial bone-marrow spectrum and soleus/gastrocnemius muscles spectrum are displayed in Fig.1 and Fig.2, respectively. Bone-marrow: a negative-significant correlation was found between L53+L52 (unsaturated fat) and age (r=-0.57, p=0.02) and between L16+L13 and age (r=-0.60, p=0.01) in female-group. L16+L13 discriminates between 36- and 36-52 female-group, and 36- and 52+ female-group. No-significant mean values metabolites differences and correlation with age were found in the male-group. No-significant difference of mean T2 was found between male and female and among age-range groups. Muscle: No-significant differences were found between fatty-acids and metabolites of males and females. Regarding the global (female+male-group) correlation with age, a significant correlation was found between EMCL15, E11, E55, I55+E55, I21 and age (Table 1, Fig.3). Cr-T2 and Cho-T2 significantly discriminate between 36- and 36-52, 52+. In general resonances-T2 of the female-group are longer than those of the male-group in all muscle resonances. Differently from literature, no significant correlation was found between IMCL, Cr, Cho, and age but we found (except for EMCL) significant T2 differences among 36+, 36-52, and 52+ group, finding higher T2 in the older group.Discussion
Bone-marrow fatty-acids quantification shows a clear sexual dimorphism with normal aging while no significant differences were found in the muscle metabolites of female and male subjects. Therefore, the observed bone-marrow fatty-acids differences during normal aging appear to be more closely related to BMD than to muscle metabolism. Muscle EMCL increases with age while IMCL remains constant. However, while the T2 values of EMCL are constant during aging, those of the IMCL show significant differences between age groups, increasing with age. As T2 is related to cell density, the results are compatible with cell microstructure degradation.Conclusions
Single-voxel-MRS and T2 measurements of metabolites in human legs could be useful for evaluating biomarkers of normal aging and sex dimorphism in the musculoskeletal system. This work suggests that instead of looking only at metabolite concentrations, tissue integrity should also be assessed with age. Indeed, any changes in metabolite concentrations may be due not so much to global changes as to a local microstructural variation in the tissues in which the metabolite is quantified.Acknowledgements
This work was supported by CNCCS ConsortiumReferences
1. Giulia Di Pietro, Manuel Scimeca, Riccardo Iundusi, Monica Celi, Elena Gasbarra, Umberto Tarantino, Silvia Capuani. Differences between muscle from osteoporotic and osteoarthritic subjects: in vitro study by diffusion-tensor MRI and histological findings. Aging Clinical and Experimental Research (2020) 32; 2489–2499.
2. Christian Cordes, Thomas Baum, Michael Dieckmeyer, Stefan Ruschke, Maximilian N. Diefenbach, Hans Hauner, Jan S. Kirschke and Dimitrios C. Karampinos. MR-Based Assessment of Bone Marrow Fat in Osteoporosis, Diabetes, and Obesity Front. Endocrinol. (2016) 7:74.
3. Laura M. Fayad, Nouha Salibi, Xin Wang, Antonio J. Machado, Michael A. Jacobs, Ronald Ouwerkerk, Baasile Okollie, David A. Bluemke, John Eng, and Peter B. Barker. Quantification of Muscle Choline Concentration by Proton MR Spectroscopy at 3 Tesla: Technical Feasibility. Am J Roentgenol. (2010) 194(1): W73–W79
4. Giulia Di Pietro, Silvia Capuani, Guglielmo Manenti, Vincenzo Vinicola, Armando Fusco, Jacopo Baldi, Manuel Scimeca, Gisela Hagberg, Marco Bozzali, Giovanni Simonetti, Umberto Tarantino. Bone marrow lipid profiles from peripheral skeleton as potential biomarkers for osteoporosis: a 1H-MR spectroscopy study. Academic Radiology (2016) 23(3);273-283.
5. Provencher, S. W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med (1993) 30, 672–679.
Figures
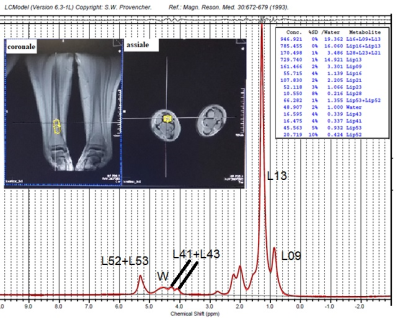
Fig.1 Single-voxel position in the tibial bone marrow of a subject and bone-marrow spectrum obtained by STEAM at TE=6ms. The principal fatty-acids resonances are highlighted.
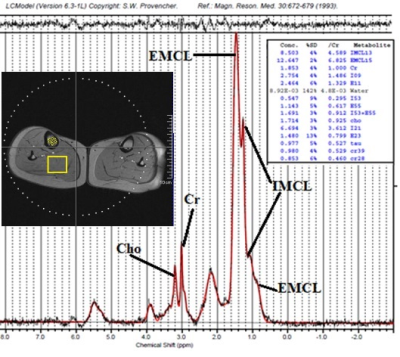
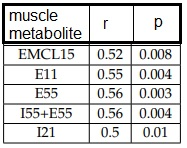
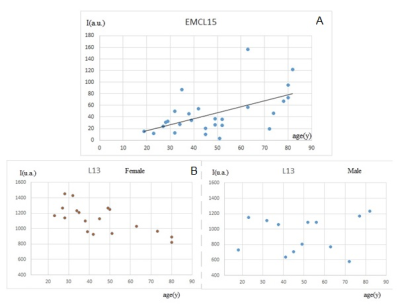
Fig. 3 A) EMCL of muscle as a function of age. A linear correlation was found between EMCL and age ( r=0.52, p=0.008) for all subjects
B) L13 of bone marrow as a function of age. A linear correlation was found between L13 and age in the female group (r=-0.60, p=0.02). Conversely, no-correlation was found in the male group.