3832
Cardiac MRI detects a reduced volume and anti-inflammatory fatty acid composition of epicardial adipose tissue in eplerenone-treated obese mice1University of Virginia, Charlottesville, VA, United States
Synopsis
Epicardial adipose tissue (EAT) volume and fatty acid composition (FAC) have significant roles in EAT-mediated coronary vascular inflammation. Using MRI of EAT volume and FAC, we tested the hypothesis that eplerenone reduces EAT volume and alters its FAC in a mouse model of obesity. We show that eplerenone significantly reduces EAT volume after 30 weeks on a high-fat high-sucrose diet compared to untreated mice. In addition, the EAT FAC is shifted from saturated fatty acid dominant in untreated mice to poly-unsaturated fatty acid dominant in eplerenone-treated mice. The results suggest that eplerenone has an anti-inflammatory role in obesity-related EAT.
Introduction
The accumulation of epicardial adipose tissue (EAT), as seen in obesity, enhances the risk of developing cardiovascular disease (CVD)1,2, potentially including coronary microvascular disease. EAT is comprised not only of adipocytes, but also immune cells, particularly macrophages3. An increased EAT volume promotes activation of the mineralocorticoid receptor (MLR)4–6 and a proinflammatory phenotype. Furthermore, the fatty acid composition (FAC) of adipose tissue contributes to the inflammatory state7,8. Specifically, saturated fatty acids (SFAs) promote an inflammatory macrophage phenotype and trigger inflammasome activation while poly-unsaturated fatty acids (PUFAs) promote an anti-inflammatory state9–11. Prior work has shown that MLR antagonism reduces fat accumulation and adipose inflammation in abdominal and epidydimal adipose tissue12,13. We hypothesized that cardiac MRI of EAT volume and FAC would show that MRL antagonism with eplerenone (EPL) reduces EAT volume and shifts the EAT FAC away from SFAs and toward PUFAs in mice fed a high-fat high-sucrose diet (HFHSD).Methods
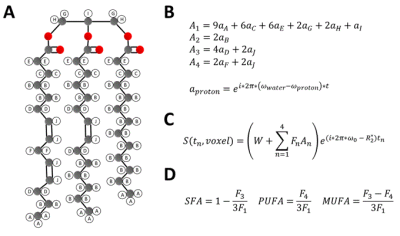
Untreated (n=5) and EPL-treated (n=5) C57Bl/6 female mice fed a HFHSD were studied. HFHSD (40% kcal fat, 40% kcal sucrose) was initiated at 10 weeks of age and continued for 30 weeks. In the treatment group, EPL (100 mg/kg/day) was added to the HFHSD chow. MRI was performed after 30 weeks of diet using a 7T system (Clinscan, Bruker) and a 35mm diameter RF birdcage coil. For EAT volume imaging, an ECG-gated three-point Dixon gradient-echo sequence using the phase-offset multiplanar method14–16 was used to acquire 6 short-axis slices from base to apex. The three echo times (TEs) were 2.5, 3.0, and 3.5ms. For EAT FAC imaging, a similar sequence was used to acquire a single mid-ventricular short-axis slice using 9 TEs equally spaced from 2.0-4.4ms. Table 1 shows a full list of parameters for both sequences. Water and fat-separated images were computed from the three-point Dixon images for all slices17, and EAT volume was calculated from the fat-only images. EAT FAC was calculated using a voxel-by-voxel Iterative Decomposition of water and fat with Echo Asymmetry and Least‐squares estimation (IDEAL) based method18. As described in Figure 1, signals from each echo were fit to a signal model accounting for the ten proton resonances present in fatty acids, four fatty acid components (F1 to F4), and a complex field map summarizing the B0 and R2* effects. The signals from the fatty acid components were then combined into SFA, PUFA, and mono-unsaturated fatty acid (MUFA) fractions within each voxel19. A t-test was used to test for differences in EAT volume and SFA, PUFA, and MUFA% between untreated and EPL-treated mice.Results
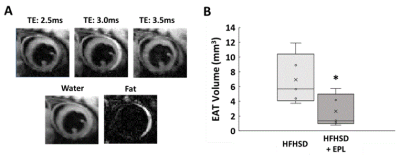
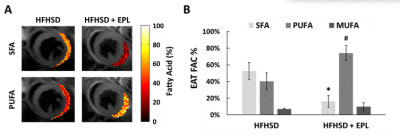
Figure 2A shows example images with water and fat in-phase (TE 3.0ms) or out-of-phase (TE 2.5ms and 3.5ms), water-only, and fat-only in a mid-ventricular slice of an untreated mouse. EAT volume was reduced in EPL-treated vs untreated mice (2.65 ± 2.20 mm3 vs 6.92 ± 3.43 mm3, p<0.05, Figure 2B). Figure 3A shows example SFA and PUFA EAT colormaps in untreated and EPL-treated mice showing changes in the FAC between the two groups. Figure 3B shows that EAT SFA% was reduced (16 ± 7% vs 53 ± 10%, p<0.01) and PUFA% increased (74 ± 9% vs 40 ± 10%, p<0.01) in EPL-treated vs untreated mice. MUFA% remained unchanged (10 ± 5% vs 7 ± 1%).Conclusion and Discussion
To the best of our knowledge, this is the first report of FAC imaging of EAT as well as of the detection of shifts in EAT FAC via drug treatment. We have previously shown that: a) HFHSD mice develop coronary microvascular dysfunction, and b) EPL in HFHSD mice preserves coronary microvascular function20. Our current results suggest that a mechanism underlying EPL-induced preservation of coronary microvascular function likely involves the reduction of EAT volume and shifting EAT fatty acid composition towards PUFAs, which is known to reduce EAT and cardiovascular inflammation. MRI of EAT volume and fatty acid composition may be indicative of EAT inflammation and represent an important imaging biomarker in coronary microvascular dysfunction and other types of inflammation-related heart disease.Acknowledgements
This work was supported by NIH NIBIB R01 EB001763.References
1. Cikim, A. S. et al. Epicardial adipose tissue, hepatic steatosis and obesity. Journal of endocrinological investigation 30, 459–464 (2007).
2. Iacobellis, G., Malavazos, A. E. & Corsi, M. M. Epicardial fat: from the biomolecular aspects to the clinical practice. The international journal of biochemistry & cell biology 43, 1651–1654 (2011).
3. Mazurek, T. et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 108, 2460–2466 (2003).
4. Guo, C. et al. Williams, GH and Adler, GK (2008) Mineralocorticoid Receptor Blockade Reverses 33 Obesity-Related Changes in Expression of Adiponectin, Peroxisome Proliferator-Activated 34 Receptor-γ, and Proinflammatory Adipokines. Circulation 117, 2253–2261 (32).
5. Hirata, A. et al. Blockade of mineralocorticoid receptor reverses adipocyte dysfunction and insulin resistance in obese mice. Cardiovascular research 84, 164–172 (2009).
6. Caprio, M. et al. Pivotal role of the mineralocorticoid receptor in corticosteroid‐induced adipogenesis. The FASEB Journal 21, 2185–2194 (2007).
7. Walker, M. E. et al. Dietary patterns influence epicardial adipose tissue fatty acid composition and inflammatory gene expression in the Ossabaw pig. The Journal of Nutritional Biochemistry 70, 138–146 (2019).
8. Vaittinen, M. et al. Interorgan cross talk between fatty acid metabolism, tissue inflammation, and FADS2 genotype in humans with obesity. Obesity 25, 545–552 (2017).
9. Wen, H. et al. Fatty acid–induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nature immunology 12, 408–415 (2011).
10. Calder, P. C. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochemical Society Transactions 45, 1105–1115 (2017).
11. Robblee, M. M. et al. Saturated fatty acids engage an IRE1α-dependent pathway to activate the NLRP3 inflammasome in myeloid cells. Cell reports 14, 2611–2623 (2016).
12. Wada, T. et al. Eplerenone prevented obesity-induced inflammasome activation and glucose intolerance. Journal of Endocrinology 235, 179–191 (2017).
13. Vecchiola, A. et al. Eplerenone implantation improved adipose dysfunction averting RAAS activation and cell division. Frontiers in Endocrinology 11, 223 (2020).
14. Berr, S. S. et al. Black blood gradient echo cine magnetic resonance imaging of the mouse heart. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 53, 1074–1079 (2005).
15. Shah, S. A. & Epstein, F. H. Multiband Cine MRI of Mouse Heart using the Phase-offset Multiplanar (POMP) Method. SCMR (2020).
16. Glover, G. H. Phase‐offset multiplanar (POMP) volume imaging: a new technique. Journal of Magnetic Resonance Imaging 1, 457–461 (1991).
17. Glover, G. & Schneider, E. Three‐point Dixon technique for true water/fat decomposition with B0 inhomogeneity correction. Magnetic resonance in medicine 18, 371–383 (1991).
18. Yu, H. et al. Multiecho reconstruction for simultaneous water‐fat decomposition and T2* estimation. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine 26, 1153–1161 (2007).
19. Berglund, J., Ahlström, H. & Kullberg, J. Model‐based mapping of fat unsaturation and chain length by chemical shift imaging—phantom validation and in vivo feasibility. Magnetic resonance in medicine 68, 1815–1827 (2012).
20. Shah, S. A. et al. Eplerenone protects against myocardial oxidative stress and preserves perfusion reserve in high fat high sucrose fed mice. SCMR 751068 (2020).
Figures