3822
Point-of-Care Brain MRI: Clinical Application in a Stroke Rehabilitation Center1Hyperfine, Guilford, CT, United States, 2Gaylord Specialty Healthcare, Wallingford, CT, United States
Synopsis
Portable, point-of-care MRI offers many new opportunities, such as scanning at the bedside or serial monitoring of a patient (i.e. daily or weekly). We demonstrate feasibility and initial results of a portable 64 mT MRI in a stroke rehabilitation setting. Six recovering stroke patients received such exams; four of them were serially monitored, each receiving four exams. Each exam consisted of T2-W, T1-W, FLAIR and DWI imaging. Different cases are highlighted to demonstrate the utility of point-of-care MRI in a rehabilitation setting.
Introduction
Magnetic resonance imaging (MRI) has become a clinical gold standard for assessing neurological disease. However, due to the siting requirements and significant costs associated with high field, superconducting systems, MRI is largely inaccessible [1] and confined to large healthcare facilities and dedicated imaging centers. Recent technological advances have pushed the barriers for conventional MRI [2-4], opening opportunities to bring MRI to the patient’s bedside. One potential benefit is serial monitoring (i.e. daily or weekly) of stroke patients in neurological intensive care units and rehabilitation centers, which could potentially influence care management and treatment plans. In this work, we present preliminary results of a portable, point-of-care MRI system in a rehabilitation center.Methods
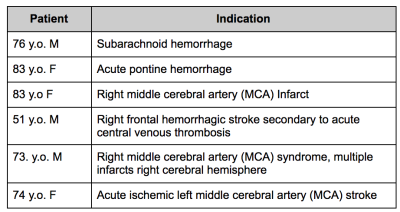
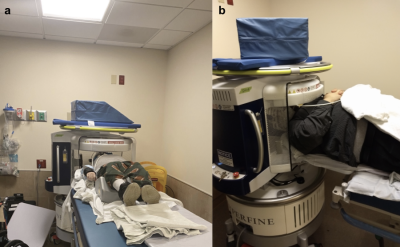
All imaging was performed under a protocol approved by the local institutional review board and written informed consent was obtained prior to imaging. Six recovering stroke patients participated in this study (Table 1). Four of the patients received a total of four scans each (where each scan was acquired 2-7 days apart), while two of the patients received one scan each.Imaging was performed on a portable SwoopTM MR system (Hyperfine, Guilford, CT) while the patient remained on the gurney (Figure 1). The permanent magnetic field has a strength of 64 mT with gradient strengths of 26 mT/m (z-axis) and 25 mT/m (x- and y-axis). The imaging protocol consisted of T1-W, T2-W, FLAIR, and diffusion-weighted imaging (DWI). Total scan time was approximately 30 minutes. All sequences were 3D, fast spin echo-based with full brain coverage and acquired in the axial plane. Resolutions for each of the scans were 1.5 x 1.5 x 5 mm3 for T1-W and T2-W scans, 1.6 x 1.6 x 5 mm3 for FLAIR and 2.4 x 2.4 x 6 mm3 for DWI. For T2-W and FLAIR imaging, two different variations were used. T2-W imaging was acquired using TE= 210.8/252 ms, TR=2000/2200, echo train length=80, number of averages=1; FLAIR imaging was acquired with TE=202.4/227.5 ms, TR=4000 ms, TI=1400 ms, echo train length=80, number of averages=1; T1-W imaging was acquired with a TE/TR/TI of 6.16/1500/300 ms, echo train length =24, number of averages =1; DWI was acquired with TE/TR=92.72/750 ms, echo train length=40, oversampling factor=5, b-value of 890 s/mm2.
Images were reviewed by a board-certified radiologist who was blinded to the patient history and medical records.
Results
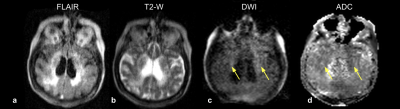
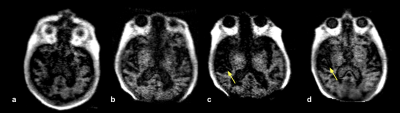
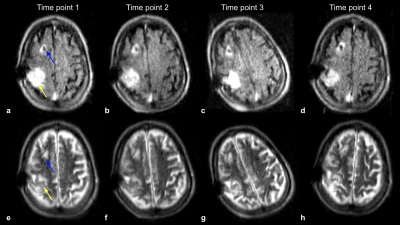
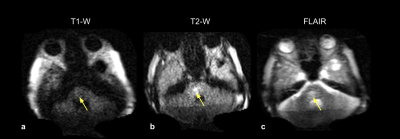
All exams were fully completed and tolerated by all subjects. Figure 2 shows the first time point of a patient with a history of a right MCA infarct. FLAIR (Fig 2a) and T2-W (Fig 2b) images demonstrate subcortical hyperintensities, with true restricted diffusion observed on the DWI (Fig 2c) and corresponding ADC map (Fig 2d). This patient received a total of four exams over the course of three weeks (Figure 3). Two weeks later (Fig 3c and Fig 3d), hypointensity in the right parietal lobe on T1-W images indicate mild encephalomalacia due to aging of infarct. Figure 4 shows a stroke patient with stable appearance of T2-W and FLAIR hyperintensity in the right parietal lobe (yellow arrow) over four time points within 19 days. Figure 5 is an example of imaging lower in the brain, highlighting a patient with a history of acute pontine hemorrhage. T1-W, T2-W and FLAIR imaging show hyperintense abnormalities in the pons indicative of vasogenic edema.Discussion and Conclusion
We demonstrate feasibility and initial results of a portable MRI in a stroke rehabilitation setting. All six cases presented brain pathologies in agreement with their presenting indication. Registration of the images would be beneficial in comparing serial scans. Future work includes correlating image findings with clinical outcomes (i.e. length of stay, speech score, grip strength score, etc.). Point-of-care MRI may offer additional applications in unprecedented settings such as rural locations, ambulances, emergency departments and neurointensive care units.Acknowledgements
No acknowledgement found.References
1. Geethanath S et al. Accessible Magnetic Resonance Imaging: A Review. Journal of Magnetic Resonance Imaging 2019; 49 (7):e65-e77.
2. Cooley CZ et al. A portable scanner for magnetic resonance imaging of the brain. Nature Biomedical Engineering 2020. https://doi.org/10.1038/s41551-020-00641-5.
3. Campbell-Washburn A. E. et al. Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI. Radiology 2019; 293: 384–393.
4. O’Reilly T et al. In vivo 3D brain and extremity MRI at 50 mT using a permanent magnet Halbach array. Magnetic Resonance in Medicine 2020; 85 (1): 495-505.
Figures