3819
A National Clinical Research MRI Quality Assurance and Quality Control Survey by NCITA1The University of Manchester, Manchester, United Kingdom, 2National Cancer Imaging Translational Accelerator (NCITA), CRUK, United Kingdom, 3Institute of Cancer Research, Sutton, United Kingdom, 4University of Cambridge, Cambridge, United Kingdom, 5Imperial College London, London, United Kingdom
Synopsis
National Cancer Imaging Translational Accelerator (NCITA) ran a survey on quality assurance (QA) and quality control (QC) procedures for human MRI scanners and associated equipment at research institutions across the UK. The survey was conducted by the NCITA QA/QC Unit, which is developing an MRI core lab to facilitate the validation and standardisation of quality-assured imaging biomarkers from first-in-human studies to the assessment of multicentre reproducibility. The results showed institutions emphasise the earlier part of the QA/QC pipeline (i.e. scanner maintenance and data acquisition QA), with fewer institutions performing comprehensive QC on acquired/analysed data or any software quality management.
Introduction
Imaging biomarkers are very challenging to translate into tools that alter clinical decision making.1 National Cancer Imaging Translational Accelerator (NCITA) is a CRUK-funded national imaging network, aiming to accelerate the standardisation and clinical translation of cancer imaging biomarkers to improve diagnosis and healthcare outcomes for patients.2 Following other initiatives in North America3-5, Europe6-8 and Asia9, this multi-institutional consortium of seven imaging centres of excellence is the first initiative in the UK.NCITA is composed of three cross-institutional units which will work in synergy to provide an integrated pipeline for the development of quality-assured cancer imaging biomarkers for clinical use.
- The Imaging Clinical Trials Unit supports and coordinates studies where the research question focuses on imaging or imaging is required to determine the primary end point.
- The Image Repository Unit is building an image repository for secure storage and sharing of imaging biomarker study data between study sites in multicentre clinical studies, as well as data processing and analysis using novel machine learning and AI tools.
- The QA/QC Unit provides robust validation, standardisation, QA and QC methods for the development of imaging biomarkers from first-in-human studies to assessing multicentre reproducibility.
Methods
On 25 August 2020, NCITA launched a National Clinical MRI Quality Assurance and Quality Control Survey to assess human MRI scanners and associated equipment at research institutions across the UK. The survey was conducted by the NCITA QA/QC Unit, which is developing an MRI core lab to facilitate the validation and standardisation of quality-assured imaging biomarkers.The survey was designed to ascertain the current status of clinical MRI scanner equipment availability, QA/QC practices and quality management procedures at research institutions across the UK to establish how the NCITA MRI core lab could best support clinical research studies using MR imaging biomarker readouts. It used a series of multiple choice questions and free-text boxes.
Representatives from all UK research institutions and centres that conduct clinical MRI imaging biomarker research studies were invited, via a number of mailing lists (BIC-ISMRM and MRIPHYSICS), to complete the survey. The deadline for completing the survey was 4 October 2020.
Results
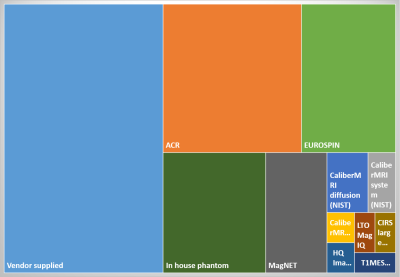
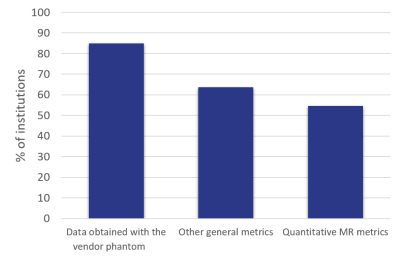
The survey closed with 33 respondents from universities (55%), NHS hospital trusts (27%) and private clinics (8%). Cancer imaging was a significant research interest at 73% of the institutions. Respondents were asked only to respond for MRI scanners where clinical research was carried out. Of those, 64% were used mainly for clinical imaging and 10% were research only. Siemens MR scanners were most common (63%), followed by GE (21%) and Philips (18%). Of those scanners, most were 3T (53%) or 1.5T (44%) and 3% were 7T. Most research institutions (85%) could cover the whole body with the coils they had available.Most institutions had research agreements on all (73%) or some (18%) of their MRI scanners and 85% of the institutions had an on-site MR physicist. Although vendor-supplied phantoms were most commonly used for QA, the ACR (SNR/geometric) and EUROSPIN (quantitative) phantoms were also popular (Figure 1). 97% of institutions had sufficient access to the clinical research MRI scanners to allow QA phantom scans to be performed. Some form of quantitative MR quality assurance was carried out at 55% of the institutions (Figure 2). Whilst 78% had standard operating procedures (SOPs) detailing equipment use, maintenance and QA, only 36% used a quality management system (QMS). Data was typically pseudo-anonymised on acquisition (43%) or on-site using anonymisation software (39%) and transferred off-site using a range of methods, with the most popular methods being electronic transfer, PACS and XNAT.
Diffusion-weighted imaging was the MR method performed at most of the institutions (84% total, 14% qualitative and 70% quantitative) and oxygen-enhanced MR at the fewest (12%, all quantitative). Quality assurance – including visual checks, phantoms and healthy volunteers – was performed on the majority of the diffusion-weighted image acquisition (78%).
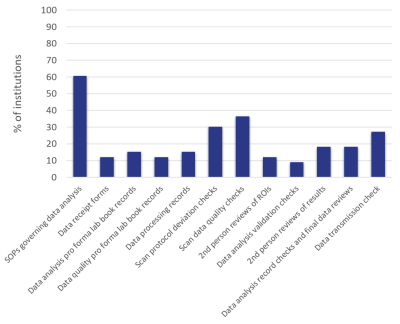
QC of the acquired data was generally underdeveloped, with only 61% of institutions having SOPs on the results of those analyses (Figure 3). Data were analysed using a range of software, primarily vendor software, imaging freeware and in-house code. Only 21% of institutions had a code review or software QMS in place for in-house code.
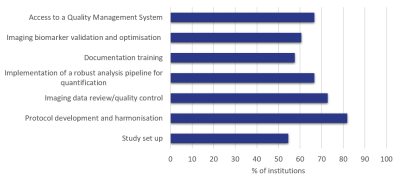
Of the proposed activities for an NCITA MR Core Lab, the most popular was Protocol Development and Harmonisation (81%), with Imaging Data Review/Quality Control (73%), Access to a QMS (67%) and Implementation of a Robust Analysis Pipeline (67%) following closely behind (Figure 4).
Conclusions
The survey revealed that the earlier sections of the QA/QC pipeline are generally well performed by clinical MR research institutions. However, QA for quantitative biomarkers is less common and the QC of the acquired data and analysis was underdeveloped. There is a little use of QMS within the clinical research imaging community, meaning multi-centre standardisation is likely to be poor and clinical translation of imaging biomarkers hindered.It is NCITA's aim to support the MR imaging community in improving all sections of the QA/QC pipeline and converting imaging biomarkers from undefined lab-based metrics into locked down, quality-assured clinical imaging toolkits.This survey provides an indication of the current status of QA/QC and will be used to focus NCITA's priorities to maximise community benefit.
Acknowledgements
We thank all NCITA partners (Imperial College London, King’s College London, University College London, University of Cambridge, University of Oxford, The Institute of Cancer Research & The Royal Marsden NHS Foundation Trust and The University of Manchester), as well as the two NCITA governance group partners (University of Glasgow and Newcastle University), and all respondents to the survey.References
1. J.P.B. O’Connor et al, Imaging biomarker roadmap for cancer studies, Nat. Rev. Clin. Oncol. 14 (2017) 169–186. https://doi.org/10.1038/nrclinonc.2016.162.
2. NCITA: www.ncita.org.uk
3. QIBA: https://www.rsna.org/en/research/quantitative-imaging-biomarkers-alliance
4. ACRIN: https://ecog-acrin.org
5. QIN: https://imaging.cancer.gov/programs_resources/specialized_initiatives/qin/about
6. EARL: http://earl.eanm.org
7. EIBALL: https://www.myesr.org/research/esr-research-committee
8. EORTC: https://www.eortc.org
9. J-QIBA: http://www.radiology.jp/j-qiba/english
Figures