3816
MRI is superior to CT for liver staging in colon cancer and should be investigated as the definitive liver staging modality in colon cancer1University of Wisconsin School of Medicine and Public Health, Madison, WI, United States, 2Georgetown University, Washington DC, MD, United States
Synopsis
Colon cancer is the 3rd most common cancer in the United States. Unfortunately, most patients present with Stage III or IV disease and disease recurrence is high despite advanced in systemic therapies. Despite evidence that gadoxetic acid-enhanced MRI is superior for the detection and diagnosis of colon cancer liver metastases, computed tomography remains the recommended staging and surveillance modality. The purpose of this abstract is to synthesize and analyze the evidence regarding the accuracy of liver MRI to stage and surveil CCLM, and to identify knowledge gaps to inform future research on staging and surveillance imaging for CCLM.
Introduction
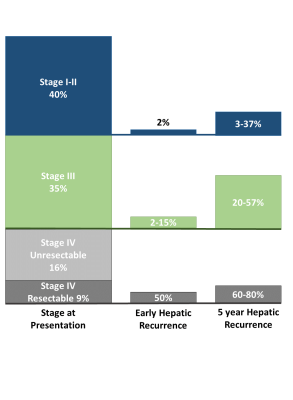
Colon cancer (CC) is the 3rd most common cancer in the United States1. Unfortunately, 35% of patients present with stage III1 and 25% present with stage IV disease2, 37.5% of whom are resectabe2-4. Even with curative intent treatment, 5 year recurrence is high: 3-37% for Stage I-II, 20-57% for stage III, and 60-80% for stage IV disease3,5,6, with 2% of Stage II, 2-15% of Stage III, and 45-50% of Stage IV recurring within 12 months of surgery (early recurrence)7-9. These rates of recurrence are summarized in Figure 1. Given the survival benefit associated with oncologic hepatic metastectomy,8-15 accurate staging and surveillance imaging diagnostics are imperative for optimized treatment. The National Comprehensive Cancer Network recommends multidetector computed tomography (MDCT) for staging and surveillance of all CC16, despite evidence that MRI is more sensitive and specific for defining CC liver metastases (CCLM)17-26. The purpose of this abstract is to synthesize and analyze the evidence regarding the accuracy of liver MRI to stage and surveil CCLM, and to identify knowledge gaps to inform future research on staging and surveillance imaging for CCLM.Diagnostic Performance of MDCT and MRI
When MDCT imaging is optimized for detecting CCLM, the sensitivity and specificity varies between 60-85%19,27,28 and 80-95%28, respectively. However, the mean sensitivity of MDCT is 65% for lesions < 1cm21-26,29 and only 8% for lesions < 0.5cm23, and further diminished by background hepatic steatosis32,35,39,40. Dissemination of MRI systems with high-performance gradients, high magnetic field strength, high channel count phased arrays coils, advanced 3D breath-hold and free-breathing dynamic T1 weighted sequences, and diffusion weighted imaging (DWI) has improved detection of even subtle CCLM. Hepatobiliary gadolinium-based contrast agents, i.e. gadoxetic acid (GA), further improve the ability of MRI to detect and characterize focal liver lesions30,31. In addition to GA-enhanced MRI, sequences recommended to detect and characterize liver lesions include fat suppressed T2-weighted fast spin-echo, 3D fat-suppressed SGRE T1-weighted, fat sensitive in- and out-of-phase (IOP) T1w SGRE, pre- and post-contrast dynamic T1w imaging during late arterial, portal venous, and delayed phases, and DWI29,32-34. The cumulative sensitivity and specificity of these sequences for diagnosing CCLM is 85-98% and 80-90%, respectively18-23,26,35-38, with a sensitivity > 90% for lesions < 1cm20. The use of GA in combination with these sequences is increasingly regarded as the standard of care for detection and characterization of CCLM20.Early Recurrence – Actual Recurrence or Occult Disease?
Early hepatic recurrence is hypothesized to represent patients with more aggressive disease biology8,39 or disease occult to imaging at staging7,9,40. In support of the latter, trials comparing diagnostic accuracy of preoperative MDCT and GA-enhanced MRI for focal liver lesions found that 17% of patients had a change in their surgical plan after MRI38. Retrospective comparison of the sensitivity of MDCT and MRI for detecting liver metastases found that MRI detected additional lesions in 40% of patients, a 6-fold increase in detection of lesions < 1 cm, and resulted in a change in treatment plan in 37% of patients19.Potential Clinical Impact of Staging MRI on Clinical Outcomes
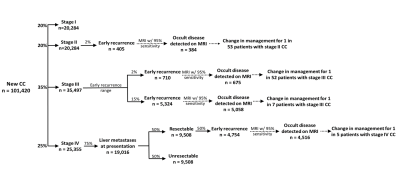
The potential impact of staging MRI is summarized in Figure 2. Assuming some proportion of “early recurrence” represents occult CCLM at CT-staging, the additive benefit of liver MRI can be estimated. Of the 101,420 CC diagnoses in 2019, 40% were Stage I-II, 35% were Stage III and 25% were Stage IV41. Assuming 2-15% of stage III disease will recur within 12 months42, 710 to 5,324 patients may have had occult hepatic metastases at staging. Assuming 95% sensitivity for MRI, occult disease may have been detected in 675 to 5,058 patients, which equates to 1 in 52 (assuming 2% early recurrence rate) or 1 in 7 (assuming 15% early recurrence rate) patients. Similarly, if early hepatic recurrence for stage IV patients who underwent curative intent surgery is 50%7-9, hepatic staging with MRI may change treatment in 4,516, or 1 in 5, of these patients.Gaps in Knowledge
The above calculations are based on limited existing clinical data on the incidence and rates of recurrence of CC. Most studies are retrospective, use data from 1980 through early 2000, do not account for systemic therapy advancements, the advent of personalized oncology targeting tumor-specific mutations57, liver hypertrophy modalities, ablation techniques, and the utilization of hepatic metastectomy. Additionally, data on the incidence of resectable stage IV disease are limited. Although synchronous liver metastases are reported to occur in 15% of all CC and are isolated to the liver in 75% of stage IV patients5, quantification of resectable hepatic disease is lacking. Studies describe that 10-37% of patients present with resectable disease5,6, however, there are no standardized criteria for determining resectability. Not accounting for these factors makes data interpretation difficult. Prospective studies that delineate the resectability of stage IV disease and the incidence and timing of hepatic recurrence are needed.Conclusion and Future Directions
Our analysis demonstrates that staging MRI may change management in 1 in 7 of Stage III patients and 1 in 5 Stage IV patients. Given the incidence of CCLM at staging, the incidence of early recurrence, and the survival benefit of hepatic metastectomy, more accurate determination of hepatic disease burden at staging and surveillance is needed. The clinical impact of improved radiologic accuracy should be assessed through randomized trials examining the impact of GA-enhanced liver MRI to conventional staging on recurrence-free and overall survival.Acknowledgements
We wish to acknowledge support from the University of Wisconsin Institute for Clinical and Translational Research. Further, we wish to acknowledge GE Healthcare who provides research support to the University of Wisconsin. Finally, Dr. Reeder is a Romnes Faculty Fellow, and has received an award provided by the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation.References
1. Society AC. Survival Rates for Colorectal Cancer. https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/survival-rates.html. Published 2019. Accessed.
2. van der Geest LG, Lam-Boer J, Koopman M, Verhoef C, Elferink MA, de Wilt JH. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis. 2015;32(5):457-465.
3. Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244(2):254-259.
4. Hackl C, Gerken M, Loss M, Klinkhammer-Schalke M, Piso P, Schlitt HJ. A population-based analysis on the rate and surgical management of colorectal liver metastases in Southern Germany. Int J Colorectal Dis. 2011;26(11):1475-1481.
5. Norstein J, Silen W. Natural history of liver metastases from colorectal carcinoma. J Gastrointest Surg. 1997;1(5):398-407.
6. Duineveld LA, van Asselt KM, Bemelman WA, et al. Symptomatic and Asymptomatic Colon Cancer Recurrence: A Multicenter Cohort Study. Ann Fam Med. 2016;14(3):215-220.
7. Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25(29):4575-4580.
8. Imai K, Allard MA, Benitez CC, et al. Early Recurrence After Hepatectomy for Colorectal Liver Metastases: What Optimal Definition and What Predictive Factors? Oncologist. 2016;21(7):887-894.
9. House MG, Ito H, Gonen M, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210(5):744-752, 752-745.
10. Petrowsky H, Gonen M, Jarnagin W, et al. Second liver resections are safe and effective treatment for recurrent hepatic metastases from colorectal cancer: a bi-institutional analysis. Ann Surg. 2002;235(6):863-871.
11. Lemke J, Cammerer G, Ganser J, et al. Survival and Prognostic Factors of Colorectal Liver Metastases After Surgical and Nonsurgical Treatment. Clin Colorectal Cancer. 2016;15(4):e183-e192.
12. Ueno S, Sakoda M, Kitazono M, et al. Is delayed liver resection appropriate for patients with metachronous colorectal metastases? Ann Surg Oncol. 2011;18(4):1104-1109.
13. Tan MC, Castaldo ET, Gao F, et al. A prognostic system applicable to patients with resectable liver metastasis from colorectal carcinoma staged by positron emission tomography with [18F]fluoro-2-deoxy-D-glucose: role of primary tumor variables. J Am Coll Surg. 2008;206(5):857-868; discussion 868-859.
14. Hallet J, Sa Cunha A, Adam R, et al. Factors influencing recurrence following initial hepatectomy for colorectal liver metastases. Br J Surg. 2016;103(10):1366-1376.
15. Vigano L, Capussotti L, Lapointe R, et al. Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol. 2014;21(4):1276-1286.
16. Network NCC. Colon Cancer: NCCN Evidence Blocks Version 2.2019-June 17, 2019. https://www.nccn.org/professionals/physician_gls/pdf/colon_blocks.pdf. Published 2019. Accessed. 17. Ward J, Robinson PJ, Guthrie JA, et al. Liver metastases in candidates for hepatic resection: comparison of helical CT and gadolinium- and SPIO-enhanced MR imaging. Radiology. 2005;237(1):170-180.
18. Chan VO, Das JP, Gerstenmaier JF, et al. Diagnostic performance of MDCT, PET/CT and gadoxetic acid (Primovist((R)))-enhanced MRI in patients with colorectal liver metastases being considered for hepatic resection: initial experience in a single centre. Ir J Med Sci. 2012;181(4):499-509.
19. Patel S, Cheek S, Osman H, Jeyarajah DR. MRI with gadoxetate disodium for colorectal liver metastasis: is it the new "imaging modality of choice"? J Gastrointest Surg. 2014;18(12):2130-2135.
20. Vilgrain V, Esvan M, Ronot M, Caumont-Prim A, Aube C, Chatellier G. A meta-analysis of diffusion-weighted and gadoxetic acid-enhanced MR imaging for the detection of liver metastases. Eur Radiol. 2016;26(12):4595-4615.
21. Donati OF, Hany TF, Reiner CS, et al. Value of retrospective fusion of PET and MR images in detection of hepatic metastases: comparison with 18F-FDG PET/CT and Gd-EOB-DTPA-enhanced MRI. J Nucl Med. 2010;51(5):692-699.
22. Seo HJ, Kim MJ, Lee JD, Chung WS, Kim YE. Gadoxetate disodium-enhanced magnetic resonance imaging versus contrast-enhanced 18F-fluorodeoxyglucose positron emission tomography/computed tomography for the detection of colorectal liver metastases. Invest Radiol. 2011;46(9):548-555.
23. Ko Y, Kim J, Park JK, et al. Limited detection of small (</= 10 mm) colorectal liver metastasis at preoperative CT in patients undergoing liver resection. PLoS One. 2017;12(12):e0189797.
24. Berger-Kulemann V, Schima W, Baroud S, et al. Gadoxetic acid-enhanced 3.0 T MR imaging versus multidetector-row CT in the detection of colorectal metastases in fatty liver using intraoperative ultrasound and histopathology as a standard of reference. Eur J Surg Oncol. 2012;38(8):670-676. 25. Kulemann V, Schima W, Tamandl D, et al. Preoperative detection of colorectal liver metastases in fatty liver: MDCT or MRI? Eur J Radiol. 2011;79(2):e1-6.
26. Scharitzer M, Ba-Ssalamah A, Ringl H, et al. Preoperative evaluation of colorectal liver metastases: comparison between gadoxetic acid-enhanced 3.0-T MRI and contrast-enhanced MDCT with histopathological correlation. Eur Radiol. 2013;23(8):2187-2196.
27. Wildi SM, Gubler C, Hany T, et al. Intraoperative sonography in patients with colorectal cancer and resectable liver metastases on preoperative FDG-PET-CT. J Clin Ultrasound. 2008;36(1):20-26. 28. Choi J. Imaging of hepatic metastases. Cancer Control. 2006;13(1):6-12.
29. Expert Panel on Gastrointestinal I, Kaur H, Hindman NM, et al. ACR Appropriateness Criteria((R)) Suspected Liver Metastases. J Am Coll Radiol. 2017;14(5S):S314-S325.
30. Imam K, Bluemke DA. MR imaging in the evaluation of hepatic metastases. Magn Reson Imaging Clin N Am. 2000;8(4):741-756.
31. Raman SS, Leary C, Bluemke DA, et al. Improved characterization of focal liver lesions with liver-specific gadoxetic acid disodium-enhanced magnetic resonance imaging: a multicenter phase 3 clinical trial. J Comput Assist Tomogr. 2010;34(2):163-172.
32. Expert Panel on Gastrointestinal I, Fowler KJ, Kaur H, et al. ACR Appropriateness Criteria((R)) Pretreatment Staging of Colorectal Cancer. J Am Coll Radiol. 2017;14(5S):S234-S244.
33. Reeder SB. State-of-the-Art Abdominal MRI: An Update. 2013 ARRS Categorical Course: Body MRI. 2013.
34. Nagle SK, Busse RF, Brau AC, et al. High resolution navigated three-dimensional T(1)-weighted hepatobiliary MRI using gadoxetic acid optimized for 1.5 Tesla. J Magn Reson Imaging. 2012;36(4):890-899.
35. Blyth S, Blakeborough A, Peterson M, Cameron IC, Majeed AW. Sensitivity of magnetic resonance imaging in the detection of colorectal liver metastases. Ann R Coll Surg Engl. 2008;90(1):25-28.
36. Marsman HA, van der Pool AE, Verheij J, et al. Hepatic steatosis assessment with CT or MRI in patients with colorectal liver metastases after neoadjuvant chemotherapy. J Surg Oncol. 2011;104(1):10-16.
37. Canellas R, Patel MJ, Agarwal S, Sahani DV. Lesion detection performance of an abbreviated gadoxetic acid-enhanced MRI protocol for colorectal liver metastasis surveillance. Eur Radiol. 2019.
38. Hammerstingl R, Huppertz A, Breuer J, et al. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol. 2008;18(3):457-467.
39. Zarour LR, Anand S, Billingsley KG, et al. Colorectal Cancer Liver Metastasis: Evolving Paradigms and Future Directions. Cell Mol Gastroenterol Hepatol. 2017;3(2):163-173.
40. Lambert LA, Colacchio TA, Barth RJ. Interval hepatic resection of colorectal metastases improves patient selection*. Curr Surg. 2000;57(5):504.
41. National Cancer Institute. Surveillance E, and End Results Program. Cancer Stat Facts: Colorectal Cancer. 2006.
42. Shah MA, Renfro LA, Allegra CJ, et al. Impact of Patient Factors on Recurrence Risk and Time Dependency of Oxaliplatin Benefit in Patients With Colon Cancer: Analysis From Modern-Era Adjuvant Studies in the Adjuvant Colon Cancer End Points (ACCENT) Database. J Clin Oncol. 2016;34(8):843-853.
Figures