3808
Setup of a cryogen-free DNP polarizer in a preclinical imaging lab1SBMI Rad. UKSH, Kiel, Germany, 2Emmy Noether Group Molecular and Metabolic MRI - M3, Freiburg, Germany
Synopsis
The setup of a cryogen-free device for dissolution dynamic nuclear polarization (dDNP) in an imaging facility is described. Fully automated procedures were used to calibrate the system and to perform hyperpolarization of pyruvic acid. Polarizations exceeding 30 % were achieved routinely within an experiment duration of less than an hour. Pitfalls are discussed and solutions proposed.
Introduction
Dissolution dynamic nuclear polarization (dDNP) is the most established technique for hyperpolarizing biomolecules for metabolic imaging. To perform dDNP, a polarizer is required, which combines several complex technologies in one specialized device. Over the last decades, several implementations were presented, some of which were commercialized (HyperSense, Oxford Instruments, SpinLab, GE). Here, we present the first experiences of the latest addition to the family, a cryogen-free dissolution polarizer for preclinical applications.Methods
A cryogen-free dDNP system was set up close to a 7 T MRI (ClinScan 70/30, Bruker) and a benchtop 1 T NMR (Spinsolve Carbon, Magritek). The main components of the polarizer are a cryogen-free, 6.7 T magnet cooled by a closed-cycle He-pump, a variable temperature insert (VTI), a microwave source, an NMR spectrometer, a dissolution module and a control software. All experiments were conducted using 30 mM trityl-radical (Polarize) in 14 M 13C-pyruvic acid (PA) placed into a custom-made cup (PEEK). Temperatures below 1.5 K were reached by evacuating a bath of helium which was liquified using the magnets’ cold finger and cycled in a close loop. A spectrometer was connected to an NMR coil inside the VTI to acquire a 63Cu, 1H or 13C signal. All procedures were controlled by a central software (Polarize; LabVIEW). The liquid-state polarization was quantified with respect to the averaged signal of the thermally polarized sample (3600 averages, flip angle 20°) and for reference also by using the C2 splitting. T1 was measured by probing the decaying polarization with 5° excitations. All quantifications were performed with a benchtop NMR and 5 mm high-throughput tubes.Results
Installation: The compact polarizer (1.6x1.1x2 m3) was easily placed in an appropriate position on its own wheels (at a distance of 3 m from the 7 T preclinical MRI and 2 m from the 1T NMR spectrometer). The He compressor for cooling the magnet was installed at a distance of 15 m and required cooling water (~4 kW) and electrical power (400 V) on-site.Setup and calibration: the magnet was evacuated to 1.6x10-2 mbar before the closed-cycle He cooler was turned on. When a temperature of 1.5 K was reached, the field was ramped up to 6.7 T in 30 min. When the desired field was approximately reached, the center frequency of the NMR console was measured using the 63Cu-signal of the coil wires (f = 75.56 MHz) and used to calculate the 1H and 13C frequencies (284.35 MHz, 71.50 MHz). The correct microwave power of 40 mW and correct position of the sample had to be measured for our system individually.
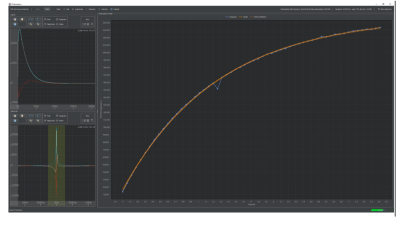
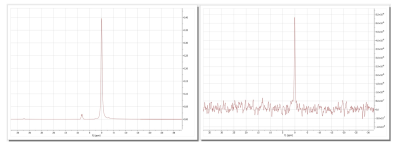
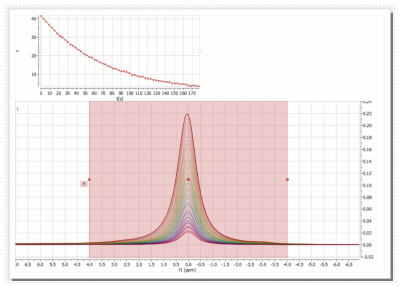
Dissolution experiments: The cup was filled with the PA Mix and lowered into the DNP cavity with a pre-programmed procedure. Once in place, DNP was initiated by turning on the microwaves and monitored by solid state, low flip angle NMR. A mono-exponential fit of the signals yielded a time constant of 1583 ± 1031 seconds for our samples. After the desired polarization was achieved, the dissolution was initiated by heating the dissolution medium at 11 bar. The sample was elevated, and the polarized solution was expelled by the dissolution medium in less than 10 s in total. Next, the sample was transferred manually to the NMR where the signal was acquired about 16 s after dissolution. With respect to the thermally polarized signal of the sample, the polarization turned out to be ca. 33 % and the C2-coupling ca. 53 %, respectively; - the reason for this difference is yet unknown. T1 in the liquid state was 66.6 ± 11.6 s. Several dDNP experiments can be run back-to-back, e.g., intervals of 1,5 h would provide a polarization of 30 %.
Discussion
The new polarizer was installed without significant hurdles. Most of the procedures were fully automated and worked smoothy as provided by the manufacturer (Polarize). The position of the sample inside the cavity turned out to be one of the most crucial factors for high and reliable polarization. An in situ (absolute) quantification of the solid-state polarization would be desirable and is currently under investigation. With a T1 of 10.5 h for 13C pyruvate in the solid state, long scan times are needed to acquire the thermally polarized signal as reference. The manual delivery to the NMR induces a variability in the polarization which can be reduced by implementing an automated setup. Most notably, the polarizer was equipped with a multitude of sensors whose data was continuously logged. This is tremendously useful for trouble shooting and reliable, reproducible experiments. The automation of all crucial processes facilitates the operation dramatically, while full access to all experimental parameters is maintained. All procedures can be adjusted to the user’s need.Conclusion
The installation, calibration and operation of the cryogen-free dDNP system was found to be straight forward and robust. In an automated experiment, taking less than an hour, a polarization of more than 30% in the liquid state was achieved. The open architecture and access to experimental parameters, on the other hand, allow the design of own experiments such as optically-induced radicals or Xenon hyperpolarization. These features make the system well suited for application studies and methodological research alike.Acknowledgements
We acknowledge support by the Emmy Noether Program “metabolic and molecular MR” (HO 4604/2-2), the research training group “materials for brain” (GRK 2154/1-2019), the DFG grant INST 257/616-1 (FUGG), the SFB “bulk-reaction” (TRR 287), the clusters of excellence “precision medicine in inflammation” (PMI 1267) and “inflammation at interfaces” (EXC 306, FOR 5042).
Kiel University and the Medical Faculty are acknowledged for supporting the Molecular Imaging North Competence Center (MOIN CC) as a core facility for imaging in vivo. MOIN CC was founded by a grant from the European Regional Development Fund (ERDF) and the Zukunftsprogramm Wirtschaft of Schleswig-Holstein (Project no. 122-09-053).
References
Müller, Christoph A., et al. "Dynamic 2D and 3D mapping of hyperpolarized pyruvate to lactate conversion in vivo with efficient multi‐echo balanced steady‐state free precession at 3 T." NMR in Biomedicine 33.6 (2020): e4291.
Ardenkjær-Larsen, Jan H., et al. "Increase in signal-to-noise ratio of> 10,000 times in liquid-state NMR." Proceedings of the National Academy of Sciences 100.18 (2003): 10158-10163.
Ardenkjær-Larsen JH, Bowen S, Petersen JR, Rybalko O, Vinding MS, Ullisch M, Nielsen NC. Cryogen-Free dissolution Dynamic Nuclear Polarization polarizer operating at 3.35 T, 6.70 T and 10.1 T. Magnetic Resonance in Medicine. 2019 Mar;81(3):2184-2194. DOI: 10.1002/mrm.27537.
Lau, Justin YC, et al. "A calibration‐based approach to real‐time in vivo monitoring of pyruvate C1 and C2 polarization using the JCC spectral asymmetry." NMR in biomedicine 26.10 (2013): 1233-1241.
Figures
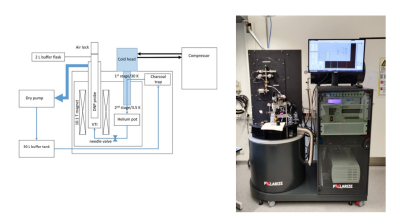
Figure 1: Scheme and Picture of dDNP system adapted from Larsen et al. (J. H. Ardenkjær‐Larsen, S. Bowen, J. R. Petersen, O. Rybalko, M. S. Vinding, M. Ullisch, N. Chr. Nielsen, Magn Reson Med. (2019), 81:2184–2194.)