3807
Early detection of Pancreatic Cancer by merging Hyperpolarized Magnetic Resonance and Artificial Intelligence1Cancer Systems Imaging, UT MD Anderson Cancer Center, Houston, TX, United States, 2UT MD Anderson UT Health GSBS, Houston, TX, United States, 3Clinical Cancer Prevention, UT MD Anderson Cancer Center, Houston, TX, United States
Synopsis
Early detection and prevention of pancreatic cancer is a modern-day challenge and there is an unmet need for non-invasive imaging markers that help identify the aggressive sub-type(s) of pancreatic ductal adenocarcinoma (PDAC) at diagnosis Our objective is to address this knowledge gap by combining hyperpolarized metabolic imaging with Artificial Intelligence (AI).
Introduction
Pancreatic cancer is one of the most aggressive types of cancers. It is difficult to detect due to its asymptomatic presentation at early stages. Therefore, there is an unmet need for non-invasive imaging markers that help identify the aggressive sub-type(s) in a pancreatic lesion at an early time point or evaluate the efficacy of therapy in pancreatic cancer. In last few years, there are two major developments that can have significant impact in developing imaging biomarkers for pancreatic cancer: I) hyperpolarized metabolic Magnetic Resonance (MR) and II) application of Artificial Intelligence (AI) on imaging pancreatic cancer. One of the most commonly used imaging biomarkers are the conversion of hyperpolarized pyruvate to lactate and alanine.1 Afterwards, the addition of Deep Learning (DL) algorithm is applied for AI to learn features from both metabolic and anatomical MR imaging modalities which has the potential for the early detection of pancreatic cancer, rather than simply monitoring tumor growth.2Methods
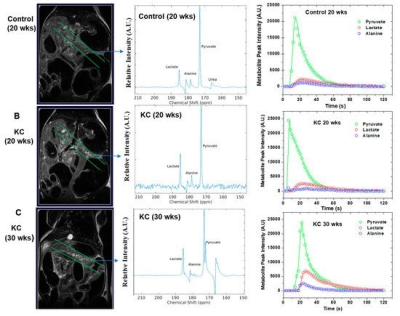
Hyperpolarized 1-13C Pyruvate MRS was employed to study the metabolic processes in genetically engineered mouse (GEM) models (P48:Cre; LSL-KRASG12D (KC)) with pre-invasive pancreatic intraepithelial neoplasia (PanIN) precursor lesions and control animals (P48:Cre or WT C57BL/6) without pancreatic lesions.. The dissolution DNP (HyperSense, Oxford Instruments) operating at 3T was utilized to hyperpolarize 1-13C pyruvate. The 13C magnetic resonance spectra of hyperpolarized 1-13C pyruvate were acquired at 7T Bruker MRI scanner. These mice were imaged at different time points in their lifespan, at 14, 21 and 28 weeks. The biochemical changes of alanine transaminase (ALT) and lactate dehydrogenase (LDH) enzyme activity were assessed. Afterwards, advanced Deep Learning (DL) techniques are implemented to develop DL model to reveal hybrid biomarkers from MRI and metabolic imaging to predict early detection of pancreatic cancer. This model is developed based on advanced Bayesian deep learning techniques and multi-modal data integration to enable uncertainty measurements and learn features from both imaging modalities to consider to improve prediction accuracy. After training the model, the learned features from multiple modalities to identify any correlation between MRI and metabolic imaging are explored that may lead to the discovery of new hybrid biomarkers with predictive values for the early detection.Results/Discussion
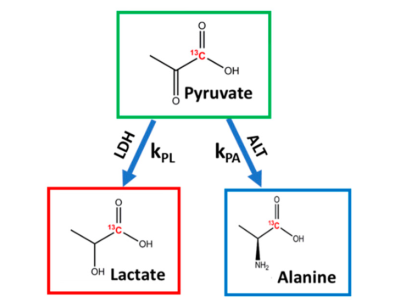
The alanine-to-lactate signal intensity ratio was found to decrease as the disease progressed from low-grade PanINs to high-grade PanINs (Figure 1). These results demonstrate that there are significant alterations of ALT and LDH activities during the transformation from early to advanced PanINs lesions. Furthermore, we demonstrated that real-time conversion kinetic rate constants (kPA and kPL) can be used as metabolic imaging biomarkers of pancreatic premalignant lesions (Figure 2). The appropriate DL combination features from MRI and metabolic imaging as complementary modalities that can lead to proper prediction of early detection in this KC GEM model.Conclusion
Findings from this emerging DL and HP-MRS techniques can be potentially translated to the clinic for detection of pancreatic premalignant lesion in high-risk populations.Acknowledgements
This research was funded in part by a grant from Pancreatic Cancer Action Network (PANCAN; 16-65-BHAT) (PKB, FM); Cancer Prevention and Research Institute of Texas (CPRIT; RP180164) (PKB) by Institutional Research Grants (PKB) and a Startup grant (PKB) from MD Anderson Cancer Center; by grants from the US National Cancer Institute (U01 CA214263, U54 CA151668 and R21 CA185536, R01 CA218004; and 1P50 CA221707-01). This work also was supported by the National Institutes of Health/NCI Cancer Center Support Grant under award number P30 CA016672.References
1. Dutta, P., Pando, S. C., Mascaro, M., et al. Early Detection of Pancreatic Intraepithelial Neoplasias (PanINs) in Transgenic Mouse Model by Hyperpolarized 13C Metabolic Magnetic Resonance Spectroscopy. International Journal of Molecular Sciences. 2020; 21(10), 3722.
2. Enriquez, J.S., Chu, Y., Pudakalakatti, S., et al (in review). Hyperpolarized Magnetic Resonance and Artificial Intelligence: Frontiers of Imaging in Pancreatic Cancer. Journal of Medical Internet Research.
Figures