3806
Effect of Radical Concentration on the Hyperpolarization of Silicon Nanoparticles using Dynamic Nuclear Polarization1Department of Physiology, Anatomy & Genetics, University of Oxford, Oxford, United Kingdom, 2Department of Physics, University of Oxford, Oxford, United Kingdom, 3Oxford Centre for Clinical Magnetic Resonance Research, University of Oxford, Oxford, United Kingdom
Synopsis
Silicon nanoparticles (SiNPs) retain enhanced polarization for several hours following hyperpolarization due to their long nuclear T1 relaxation time and are therefore attractive candidates for use as MRI contrast agents. However, “bare” SiNPs show low signal enhancement and require the addition of exogenous radicals to reach sufficient signal enhancements for MRI. Here, the addition of two radicals (Finland trityl and TEMPO) and the effect of their concentration on SiNP build-up and decay properties were investigated. Optimising SiNP polarization characteristics is necessary if their clinical translation as targeted hyperpolarized contrast agents is to be achieved.
Introduction
The use of hyperpolarized contrast agents has helped overcome one of the main limitations of Magnetic Resonance Imaging (MRI), that being low sensitivity. Dynamic Nuclear Polarization (DNP) has evolved as a key tool in the production of hyperpolarized contrast agents and is most frequently used with 13C based samples for clinical metabolism studies [1]. One limitation of 13C, however, is the rapid rate with which its hyperpolarization decays. Consequently, there is increasing interest in using nuclei with longer signal enhancement lifetimes, such as silicon (29Si) [2].The slower signal decay (i.e. longer T1 relaxation times) of 29Si nanoparticles (SiNPs) hyperpolarized by DNP provides an imaging window of approximately two hours which is significantly longer than that of 13C (60-120 s) [3]. As a result, SiNPs have the potential to be used as hyperpolarized contrast agents but progress in their preclinical development has been impacted due to low signal enhancement achieved during the DNP process – the hypothetical cause of which is the low number of endogenous electronic defects.
To overcome this, in 2018 Hu et al took advantage of the flexible surface chemistry of SiNPs and introduced a method which increased the number of free electrons available to polarize SiNPs through the addition of the TEMPO radical. Their findings showed signal enhancement sufficient for in vivo MR imaging [4] and the work presented here extends on these findings by comparing the effect of different exogenous free radicals on the signal enhancement and signal decay of SiNPs.
Methods
Samples consisted of ~38 mg 30-50 nm SiNPs (US Research Nanomaterials Inc, Houston, TX USA) suspended in 130 µL of 1:1 dimethyl sulfoxide (DMSO) and deuterium oxide (D2O) and mixed with varying radical concentrations (15 mM – 60 mM) of either Finland trityl or TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy). Mixtures were vortexed and sonicated until a liquid formed which was then pipetted into a small PEEK sample cup.Each sample was inserted into a protype hyperpolarizer at 1.3 K, and 3.35T/93 GHz for DNP. Frequency sweeps (93.76–94.25 GHz @ 100 mW, 5 min build-up per frequency) acquired for each sample were used to determine the optimal polarization frequency. Polarization build-up at the optimal frequency was subsequently monitored every five minutes for five hours using a low flip-angle readout. Once the polarization build-up curve had plateaued, the microwave source was switched off and the decay of polarization immediately monitored to assess the signal decay rate. Acquired spectra were analysed in jMRUI and the data fitted to mono-exponential functions, correcting for the effects of the applied excitation pulses, to yield the build-up time constants, build-up amplitudes and decay time constants for each sample.
Results
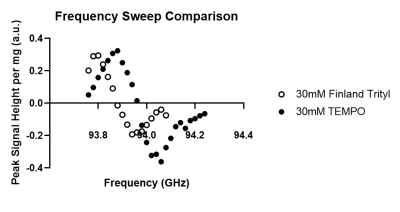
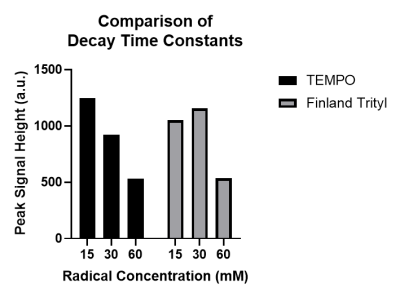
Whilst both radicals showed similar frequency sweep patterns (figure 1), the use of the Finland radical lead to a narrower enhancement peak with a lower optimal frequency. Example build-up and decay profiles for both radicals are shown in figure 2, with the quantified build-up and decay properties shown in figures 3-5. The dependencies of build-up amplitudes and build-up time constants on radical concentration followed the same trend for both Finland and TEMPO containing samples; build-up amplitudes decreased, and build-up time constants shortened with increasing radical concentration. TEMPO containing samples exhibited larger build-up amplitudes and longer build-up time constants compared to samples containing the same concentration of Finland. Additionally, for samples that contained TEMPO, the decay time constant shortened with increasing radical concentration whereas, for samples containing Finland, the longest decay time constant was recorded at 30 mM concentration. Overall, 15 mM TEMPO gave the largest build-up amplitude, longest build-up time constant (>13 hours), and longest decay time constant (>20 hours).Discussion
Greater radical concentrations mean a larger number of exogenous free radicals (which act as polarization centres during DNP) near SiNPs and thus, a greater electron polarization available for transfer to 29Si nuclei. This results in a rapid build-up of polarization which reaches saturation sooner than samples containing less radical.Shorter build-up times are desirable and so greater radical concentration would appear to be optimal, however, there is a trade off with build-up amplitude which decreases with increasing radical concentration. Greater concentrations of radical may also increase the rate of signal decay due to radicals close to 29Si nuclei acting as relaxation centres post-DNP. When radicals are at sufficient distances from 29Si nuclei, slower signal decay occurs via spin diffusion. From the data presented here the optimal radical concentration for hyperpolarizing SiNPs would appear to be 15mM for Finland and 30mM for TEMPO. These concentrations would minimise the build-up time (~2 hours) without compromising too much on the build-up amplitude whilst maintaining a decay time constant of >15 hours.
Conclusion
The clinical potential of SiNPs as targeted, and potentially drug loaded, hyperpolarized contrast agents has been widely acknowledged. However, further optimisation of SiNP polarization characteristics is required before this potential is realised. The presented work further investigates a method which increases the intrinsically low signal enhancement of "bare" SiNPs. Understanding the impact different structures of radicals have on polarization characteristics could give focus to the optimisation of SiNPs as clinical hyperpolarized contrast agents by informing the choice of radical for in vivo toxicity and specificity studies, for example.Acknowledgements
This work was supported by funding from the Engineering and Physical Sciences Research Council (EPSRC) and Medical Research Council (MRC) [grant number EP/L016052/1]
The authors would like to acknowledge the members of the Cardiovascular Metabolism Research Group (DPAG, University of Oxford), with special thanks to Vicky Ball.
References
[1] Chaumeil, M.M., Najac, C. and Ronen, S.M., 2015. Studies of metabolism using 13C MRS of hyperpolarized probes. In Methods in enzymology (Vol. 561, pp. 1-71). Academic Press
[2] Cassidy, M. C., Chan, H. R., Ross, B. D., Bhattacharya, P. K., & Marcus, C. M. (2013). In vivo magnetic resonance imaging of hyperpolarized silicon particles. Nature nanotechnology, 8(5), 363-368
[3] Aptekar, J.W., Cassidy, M.C., Johnson, A.C., Barton, R.A., Lee, M., Ogier, A.C., Vo, C., Anahtar, M.N., Ren, Y., Bhatia, S.N. and Ramanathan, C., 2009. Silicon nanoparticles as hyperpolarized magnetic resonance imaging agents. ACS nano, 3(12), pp.4003-4008.
[4] Hu, J., Whiting, N. and Bhattacharya, P., 2018. Hyperpolarization of Silicon Nanoparticles with TEMPO Radicals. The Journal of Physical Chemistry C, 122(19), pp.10575-10581.
Figures