3805
Dramatic Effect of Pulse Length and Bandwidth on the Efficiency of Pulsed Spin-Order-Transfer Sequences at High Field.1Radiology, Uniiklinik Freiburg, Freiburg, Germany, 2radiology, Uniklinik Freiburg, Freiburg, Germany, 3radiology, Uniklinik freiburg, freiburg, Germany, 4hyperpolarization, Kiel university, Kiel, Germany, 5radiology, Uniklinik Freiburg, freiburg, Germany, 6Radiology, Uniklinik Freiburg, freiburg, Germany
Synopsis
Para-Hydrogen Induced Polarization (PHIP) is a promising cost- and time-efficient technique for highly-sensitive metabolic MRI. We recently demonstrated PHIP with 13C-polarizations of 25% inside MRI systems at high field using spin-order transfer (SOT) sequences. Here, we show that SOT is extremely sensitive to limited proton-RF bandwidth available in MRI setups. This effect is crucial for hyperpolarization of metabolic agents like acetate and pyruvate; and for PHIP in human MRI setups, dedicated hardware may be required. Still, high-field PHIP is promising as the setup is inexpensive and simple and requires no transport of the agents to the MRI system is needed.
INTRODUCTION
Hyperpolarization (HP) allows a signal enhancement that opens promising perspectives for MRI-based diagnostics, e.g. metabolic MRI with highly increased sensitivity. In contrast to Dissolution Dynamic Nuclear Polarization (dDNP), ParaHydrogen (pH2) Induced Polarization (PHIP) is a much faster and cost-efficient method to produce hyperpolarized Contrast Agents (CA). We recently demonstrated Synthesis Amid the Magnet Bore, which Allowed Dramatically Enhanced Nuclear Alignment (SAMBADENA)1.With SAMBADENA the HP (hydrogenation and Spin-Order Transfer (SOT)) and application of CA are performed in the bore of the same MRI system within seconds and without transferring CA’s to the magnet. This technique is of particular interest, because PHIP has recently been demonstrated for a substantial spectrum of promising metabolic CA’s, including pyruvate, acetate and 2,3,4,5 using pulsed SOT sequences, 13C-HP of ≈60 or ≈20% has been demonstrated for esters of acetate and pyruvate, respectively, at high field using an NMR-spectrometer setup6. However, the increased frequency-spread due to chemical shift (δ) at high field poses new physical challenges to SAMBADENA arising from limited radiofrequency (RF) power and quadratic decrease of RF excitation due to large bore. For instance, the pH2-nascent protons – which are added to a precursor by hydrogenation to incorporate the spin-order to the CA – possess differences of δ of ≈1 or ≈3ppm (≈300 or 900Hz at 7T) for the established PHIP-CA’s cinnamyl-pyruvate or ethyl-acetate, and phosphor-lactate, respectively.
Here, we study the effect of pulse length and asymmetric excitation of pH2-nascent protons during SOT for the first time.
METHOD
SetupThe setup and experiment were described in detail before (Fig. 1)1. In short, a preclinical 7T MRI system was used along with a transmit-receive-1H-13C-volume resonator (Inner diameter: 7.1cm). HP took place within a custom-made polysulfone reactor placed in the isocenter of the MRI setup. 1H flip angles were calibrated using automated routines from the vendor with 1mL H2O filled into the reactor. 13C flip angles were calibrated manually using a 13C-enriched model solution (4M 1-13C-sodium acetate in 0.5mL H2O, 99% 13C, CAS: 23424-28-4; doped with 7.5 µL gadolinium, T1=832ms).
Experiments
Samples were prepared in H2O with 2mM water-soluble rhodium-based catalyst and 2mM precursor (2,2,3-2H-hydroxyethyl-1-13C-acrylate to form 2,2,3-2H-hydroxyethyl-1-13C-propionate (HEP); pH2-derived 1H at δ=0.94ppm and δ=2.27ppm; ∆δ≈1.3ppm). The PH-INEPT+ sequence was used for SOT (Fig. 2)7. Hydrogenation took place at 7T, 80°C, and ≈5 bar using H2 gas with ≈90% pH28.
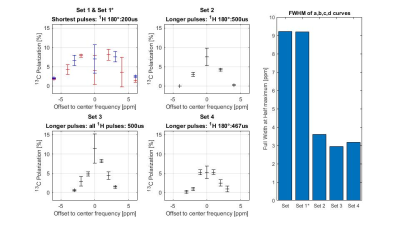
13C-HP was measured as function of the frequency offset of the 1H-RF excitation from the center of the pH2-nascent 1H at 1.6 ppm for four different settings of the 1H and 13C RF pulse lengths (Table 1).
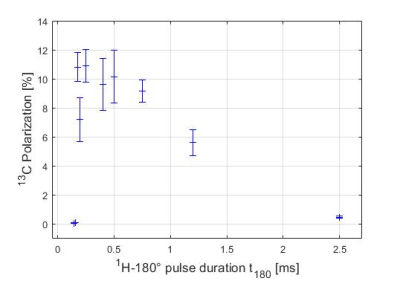
In another experiment, 13C-HP was measured as function of the 1H pulse power level (9 values between 2.65 and 775W (maximum-power output)) while the 1H-RF center was set to 1.6ppm. Thereby, the length of the pulses required for 45, 90 and 180° flip angle was changed with t180=2·t90=4·t45. The 13C-RF power was set to the maximum output of the MRI setup (829W), corresponding to 233 or 467ms for a 90 or 180° pulse, respectively.
In all settings, the 13C-RF center was set to δ of 1-13C-HEP (≈179ppm)
Quantification of HP
An exponential-decay function (10Hz) was multiplied to the time-domain data, followed by Fourier transformation, phase and baseline correction (TopSpin 4.0, Bruker). The frequency-domain HP signals were integrated using custom-written software (Matlab R2020b, MathWorks, USA). HP was quantified by comparing HP signals with signal acquired from the above-mentioned, thermally-polarized model solution8.
RESULTS
When the 1H-RF center was moved from 1.6ppm, a prominent decrease of HP was observed (Fig. 3). This effect was less dramatic, when shorter 1H pulses were applied. To a first approximation of the apparent 1H-bandwidth of the PH-INEPT+ sequence we fitted a Gaussian to the data and determined the Full-Width-at-Half-Maximum (FWHM; Fig. 3e). For instance, when all pulses (1H and 13C) were set to 500µs, FWHM≈2.94ppm was determined compared to FWHM≈9.22ppm for set1 (1H-45,90,180°: 50,100,200µs; 13C-90,180°: 200,467µs; Table 1).Along with the 1H power level, the 13C-HP increased from ≈0.5% (t180=2.5ms) to (11±1)% (t180=170µs; Fig. 4). Note that similar HP was observed for power levels between 66 and 540W (corresponding to t180=170µs to 500µs). However, for higher powers, the HP dropped remarkably likely because of power limitations of the RF hardware (0.11% for t180=160s).
DISCUSSION
Naturally shorter pulses correspond to a larger frequency bandwidth. However, while the nominal bandwidth of a RF block pulse is often stated with inverse pulse length for a 500 or 200µs pulse), we found the PH-INEPT+ sequence being surprisingly sensitive to 1H off-resonance. While PH-INEPT+ only consists of four 1H-pulses, other SOT sequences require more pulses or 1H-decoupling (e.g. ESOTHERIC sequence6). Moreover, the pH2-nascent protons in HEP have ∆δ=1.3ppm but other PHIP-molecules feature larger ∆δ. We assume that the 1H-pulse length will play an important role if SAMBADENA were used for human applications in future. Moreover, to reproduce the remarkably high PHIP 13C-HP of metabolites achieved by Glöggler and coworkers in an NMR spectrometer9, dedicated RF hardware or composite-pulse schemes may be required.CONCLUSION
We demonstrated that the 1H-pulse bandwidth significantly influences the efficiency of high-field SOT. To provide highly polarized metabolic CAs with SAMBADENA, this effect may be crucial and will have to be considered.Acknowledgements
Andreas Schmidt and Stephan Berner want to acknowledge funding support of the Research Commission of the University Medical Center Freiburg (SCHM2146-20) and the German Consortium for Translational Cancer Research (DKTK). Andreas Schmidt further acknowledges support by the Heinrich-Böll foundation (P131623), the Emmy Noether Program of the German Research Foundation (DFG, HO 4604/2‐1, 4604/2‐2) and the Wayne State University (Faculty Postdoctoral Award together with Prof. Eduard Chekmenev, Detroit, US; 177805).References
1 Schmidt, Andreas B., Stephan Berner, Moritz Braig, Mirko Zimmermann, Jürgen Hennig, Dominik von Elverfeldt, and Jan-Bernd Hövener. “In Vivo 13C-MRI Using SAMBADENA.” PLOS ONE 13, no. 7 (12 2018): e0200141. https://doi.org/10.1371/journal.pone.0200141.
2 Reineri, Francesca, Tommaso Boi, and Silvio Aime. “ParaHydrogen Induced Polarization of 13 C Carboxylate Resonance in Acetate and Pyruvate.” Nature Communications 6, no. 1 (January 5, 2015): 5858. https://doi.org/10.1038/ncomms6858.
3 Shchepin, Roman V., Aaron M. Coffey, Kevin W. Waddell, and Eduard Y. Chekmenev. “Parahydrogen Induced Polarization of 1-13C-Phospholactate-D2 for Biomedical Imaging with >30,000,000-Fold NMR Signal Enhancement in Water.” Analytical Chemistry 86, no. 12 (June 17, 2014): 5601–5. https://doi.org/10.1021/ac500952z.
4 Cavallari, Eleonora, Carla Carrera, Silvio Aime, and Francesca Reineri. “13C MR Hyperpolarization of Lactate by Using ParaHydrogen and Metabolic Transformation in Vitro.” Chemistry – A European Journal 23, no. 5 (2017): 1200–1204. https://doi.org/10.1002/chem.201605329.
5 Cavallari, Eleonora, Carla Carrera, Matteo Sorge, Gisèle Bonne, Antoine Muchir, Silvio Aime, and Francesca Reineri. “The 13 C Hyperpolarized Pyruvate Generated by ParaHydrogen Detects the Response of the Heart to Altered Metabolism in Real Time.” Scientific Reports 8, no. 1 (May 30, 2018): 8366. https://doi.org/10.1038/s41598-018-26583-2.
6 Korchak, Sergey, Shengjun Yang, Salvatore Mamone, and Stefan Glöggler. “Pulsed Magnetic Resonance to Signal‐Enhance Metabolites within Seconds by Utilizing Para‐Hydrogen.” ChemistryOpen 7, no. 5 (May 8, 2018): 344–48. https://doi.org/10.1002/open.201800024.
7 Haake, Mathias, Johannes Natterer, and Joachim Bargon. “Efficient NMR Pulse Sequences to Transfer the Parahydrogen-Induced Polarization to Hetero Nuclei.” Journal of the American Chemical Society 118, no. 36 (January 1996): 8688–91. https://doi.org/10.1021/ja960067f.
8 Schmidt, Andreas. “Liquid-State Nuclear Hyperpolarization without a Polarizer : Synthesis amid the Magnet Bore Allows a Dramatically Enhanced Nuclear Alignment,” 2020.
9 Korchak, Sergey, Salvatore Mamone, and Stefan Glöggler. “Over 50 % 1H and 13C Polarization for Generating Hyperpolarized Metabolites—A Para-Hydrogen Approach.” ChemistryOpen 7, no. 9 (2018): 672–76. https://doi.org/10.1002/open.201800086.
Figures
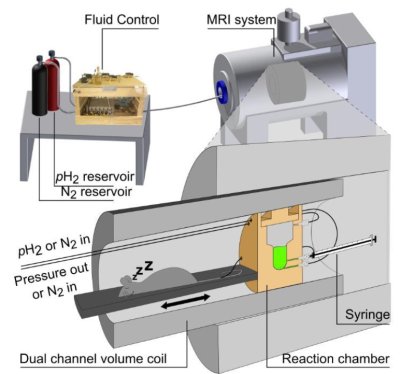
Figure 2 - Schematic of the PH-INEPT+ SOT sequence. The representation shows RF pulse power against time for the 1H and 13C channels. RF block pulses with different flip angles (45, 90 or 180°) were played out interleaved by times of free evolution (t1 and t2) to convert the 1H spin order of the pH2 into 13C hyperpolarization. The PH-INEPT+ sequence is well suited for high fields because it is specialized for SOT in weakly-coupled spin systems (where ∆δ is much larger than their J-coupling).