3804
Hyperpolarized in-vivo Metabolic Imaging at 14.1T: dDNP Cryogenic Insert Redesign and Implementation1Geneva School of Health Sciences, HES-SO University of Applied Sciences and Arts Western Switzerland, Geneva, Switzerland, 2Laboratory of Functional and Metabolic Imaging, EPFL (Swiss Federal Institute of Technology in Lausanne), Lausanne, Switzerland, 3Department of Health Technology, Center for Hyperpolarization in Magnetic Resonance, Technical University of Denmark, Kongens Lyngby, Denmark, 4Image Guided Intervention Laboratory, University of Geneva (UNIGE), Geneva, Switzerland
Synopsis
Hyperpolarized MR studies of localized animal disease models, specifically our studies of cerebral metabolism after transient ischemia in a mouse model of stroke, are greatly improved by implementation of spatially resolved measurements. The latter allows unfolding specific metabolic alterations in core, penumbra and peripheral tissues taking place after ischemia. To achieve higher specificity, the performances of a DNP polarizer were improved by installing a custom fluid path dissolution system to achieve higher polarization levels. In parallel, we implemented a dynamic spiral acquisition sequence to enable time-resolved MR metabolic imaging. Herein, we present our preliminary in-vivo proof of principle.
Introduction
Dissolution dynamic nuclear polarization1 (dDNP) is a method for boosting by several orders of magnitude the sensitivity of MR imaging and spectroscopy. The technique’s main feature entails the polarization of MR-active substrates beyond thermal equilibrium. This hyperpolarized (HP) state gifts MR with unprecedented temporal resolution, enabling non-invasive in-vivo assessment of metabolic conversion.To this end, we previously employed global HP MRS to improve our understanding of the metabolic changes in a mouse model of stroke, as well as the neuroprotective mechanisms following injection of HP lactate and pyruvate at a therapeutic dose2,3. The specific regional patterns of the response to a stroke insult require an appropriate localization of those metabolic processes. Given our experimental setup, a better outcome of the experiment required technological improvements on both the metabolic imaging acquisition side, and the DNP polarizer performance.
We upgraded an existing dedicated wet polarizer by adapting the recently developed custom fluid path4–6 (CFP), and evaluated its performances. In parallel, we implemented a spiral IDEAL acquisition scheme for dynamic imaging of the metabolic processes following injection of pyruvate or lactate at therapeutic doses, and performed initial in-vivo testing on healthy animals.
Methods
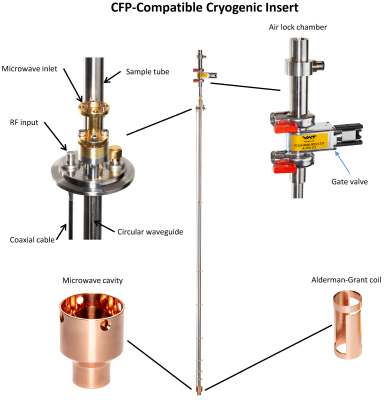
MR experiments were performed in a 14.1T/260mm horizontal bore magnet (Magnex Scientific) and 1000mT/m shielded gradient set (Resonance Research) interfaced to a BioSpec Advance NEO MRI console (Bruker).Polarizer upgrade: A 5T/1.2K wet DNP polarizer7,8 was retrofitted with a new cryogenic insert (Fig.1) to accommodate a CFP4–6.
Hyperpolarization: Neat [1-13C]pyruvic acid (PA) (Sigma Aldrich) was doped with 15mM of OX063 radical (Albeda Research) and loaded at the bottom of the sample vial. 5.0µl and 20.0µl of sample were used for in-vitro and in-vivo experiments respectively. A stoichiometric amount of a NaOH 10M was frozen on the vial's walls to neutralize the acid during dissolution. PA was hyperpolarized at the positive DNP enhancement maximum (139.86GHz) for 2h-3h. The sample was dissolved in a physiological buffer preheated at 180°C and pushed to a separator/infusion pump7 inside the 14.1T scanner bore, over a 6m line.
Polarization measurements: The HP signal was measured in the separator/infusion pump9 (FA=10°, TR=3s). The liquid-state hyperpolarization value reaching the scanner was estimated by computing the ratio between the first HP signal and the thermal equilibrium signal (1024 averages, TR=5s, after adding 1mM of Gadobutrolum). This procedure was performed three times before and after the upgrade.
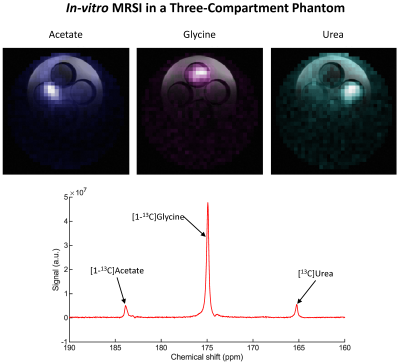
MRSI protocol: A 2D IDEAL Spiral CSI10 sequence was developed on the BioSpec console. Image reconstruction was implemented in MATLAB with chemical-shift modelling reconstruction10,11 prior to spatial gridding reconstruction. Acquisition parameters were optimized to minimize the effects of strong B0 inhomogeneity and short T2* at ultra-high field. The protocol was tested on a 3-compartment phantom with [1-13C]acetate, [1-13C]glycine and [13C]urea.
Cerebral HP MRSI: One C57BL6/J male mouse (29.8g) was scanned to test the MRSI protocol. The signal was acquired with a 1H quadrature/13C linear (⌀14/11mm) surface coil placed on the animal’s head. Field map-based shimming12 optimized B0 homogeneity. A 300µl bolus of 82mM HP pyruvate was injected into the mouse. The acquisition was triggered at 3s post-infusion.
Results
With the CFP, the upgraded DNP polarizer yielded a liquid-state polarization of (33.3±1.6)% with a mono-exponential build-up time constant of (2745±63)s for PA, which were higher and faster compared to the original dissolution wand set-up (Fig.2). The liquid-state T1 at 14.1T was (46.7±1.7)s.In-vitro testing of the MRSI protocol successfully resolved the different chemical shifts of the 3-compartment phantom (Fig.3).
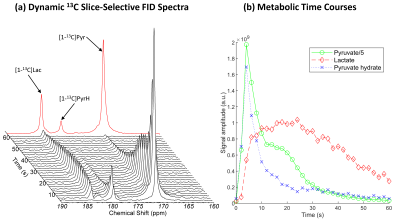
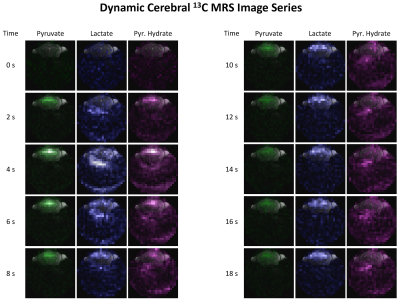
In animal experiments, (2.5±0.2)ml of (82±4)mM PA at pH=7.50±0.14 were collected in the separator (n=4). Upon infusion, spectra of slice-selective free-induction decays indicate lactate labelling from pyruvate (Fig.4). Dynamic spectroscopic images show the influx of pyruvate and pyruvate hydrate from the vasculature followed by a diffuse labelling of lactate (Fig.5). The nominal in-plane resolution was 0.92x0.92mm2. To fully evaluate the protocol performances, no additional zero filling, filtering or masking was applied.
Discussion
The CFP offers better hyperpolarization performances compared to the original design, achieving 52% higher and 30% faster polarization when PA and trityl radical are employed. This could be related to the higher microwave power density achieved by reducing the microwave cavity volume by 54%. Moreover, compared to the traditional dissolution wand system, the CFP provided a better reproducibility in both the concentration and pH level of the pyruvate solution. The improved accuracy in dissolution performance will minimize the experimental variability for animal studies.Initial experiments successfully provided submillimetric dynamic spectroscopic images of cerebral [1-13C]pyruvate metabolism. However, the acquisition presented a poor dynamic range and sensitivity: the influx of pyruvate prevented imaging lactate in the first four images, and the pyruvate hydrate signal was insufficient beyond the fifth repetition. Additionally, the surface coil could not achieve whole-brain coverage. Further improvements are underway to refine the sensitivity and dynamic range, to match the requirement specifications of our mouse model of stroke.
Conclusion
In this work, we upgraded an existing wet DNP polarizer to accommodate a CFP. This technological improvement enabled better polarization performances to the benefit of MR applications. Despite our modest results, we also show our preliminary implementation of dynamic HP MRSI at ultra-high field, which will bring deeper insights for in-vivo studies after further optimizations.Acknowledgements
This study is supported by the Swiss National Science Foundation (170155 to JN. Hyacinthe, 190547 and 193276 to A. Capozzi). The authors gratefully thank Drs. Mario Lepore, Analina Hausin and Stefan Mitrea for their assistance in the animal preparation, Yves Pilloud and Dr. Hikari Yoshihara for their technical advices, Dr. Mor Mishkovsky for taking care of the animal authorization, as well as the Center for Biomedical Imaging of the University of Lausanne, EPFL, University of Geneva, Geneva University Hospitals, Lausanne University Hospital, and the Leenaards and Louis-Jeantet Foundations.References
1. Ardenkjaer-Larsen, J. H. et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. U. S. A. 100, 10158–63 (2003).
2. Hyacinthe, J.-N. et al. Evaluating the potential of hyperpolarised [1-13C] L-lactate as a neuroprotectant metabolic biosensor for stroke. Sci. Rep. 10, 5507 (2020).
3. Lê, T. P. et al. Metabolism of the hyperpolarized neuroprotective agents [1-13C] lactate and [1-13C] pyruvate in a mouse model of transient ischemic stroke. in Proc. Intl. Soc. Mag. Reson. Med. 28 0689 (2020).
4. Capozzi, A., Karlsson, M., Petersen, J. R., Lerche, M. H. & Ardenkjaer-Larsen, J. H. Liquid-State 13C Polarization of 30% through Photoinduced Nonpersistent Radicals. J. Phys. Chem. C 122, 7432–7443 (2018).
5. Pinon, A. C., Capozzi, A. & Ardenkjær-Larsen, J. H. Hyperpolarized water through dissolution dynamic nuclear polarization with UV-generated radicals. Commun. Chem. 3, 1–9 (2020).
6. Pinon, A. C., Capozzi, A. & Ardenkjær-Larsen, J. H. Hyperpolarization via dissolution dynamic nuclear polarization: new technological and methodological advances. Magn. Reson. Mater. Physics, Biol. Med. (2020) doi:10.1007/s10334-020-00894-w.
7. Comment, A. et al. Design and performance of a DNP prepolarizer coupled to a rodent MRI scanner. Concepts Magn. Reson. Part B 31B, 255–269 (2007).
8. Cheng, T., Capozzi, A., Takado, Y., Balzan, R. & Comment, A. Over 35% liquid-state 13C polarization obtained via dissolution dynamic nuclear polarization at 7 T and 1 K using ubiquitous nitroxyl radicals. Phys. Chem. Chem. Phys. 15, 20819–20822 (2013).
9. Cheng, T. et al. Automated transfer and injection of hyperpolarized molecules with polarization measurement prior to in vivo NMR. NMR Biomed. 26, 1582–1588 (2013).
10. Wiesinger, F. et al. IDEAL spiral CSI for dynamic metabolic MR imaging of hyperpolarized [1- 13C]pyruvate. Magn. Reson. Med. 68, 8–16 (2012).
11. Reeder, S. B., Brittain, J. H., Grist, T. M. & Yen, Y. F. Least-squares chemical shift separation for 13C metabolic imaging. J. Magn. Reson. Imaging 26, 1145–1152 (2007).
12. Kanayama, S., Kuhara, S. & Satoh, K. In vivo rapid magnetic field measurement and shimming using single scan differential phase mapping. Magn. Reson. Med. 36, 637–642 (1996).
Figures