3803
Slowing T1 relaxation of hyperpolarized [2-13C]pyruvate with deuterium enrichment1GE Healthcare, Toronto, ON, Canada, 2Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 3Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada
Synopsis
The effect of deuterium enrichment on T1 relaxation was investigated for hyperpolarized [2-13C]pyruvate. The T1 increased by 10s at 3T with deurterium enrichment on the sample molecule. The T1 further increased by almost 30s when the hyperpolarized sample was dissolved using D2O instead of H2O. Deuterium enrichment of the substrate and the dissolution media has the potential to significantly increase the available non-equilibrium polarization available at the time of injection for dissolution DNP experiments.
Introduction:
While [1-13C]pyruvate is the molecule used the most for dynamic nuclear polarization (DNP) based hyperpolarized 13C MR studies to date, pyruvate with 13C enrichment at the C2 position or both C1 and C2 positions have been explored in both pre-clinical and human studies (1-3). With HP [2-13C]pyruvate, it may be possible to investigate TCA cycle turnover, in addition to pyruvate dehydrogenase flux. However, [2-13C]pyruvate has a shorter T1 than [1-13C]pyruvate, resulting in lower polarization by the time the substrate reaches the tissue of interest, making imaging of the relatively small metabolic product resonances such as [5-13C]glutamate more difficult. While deuterium enrichment strategies have been utilized to lengthen the T1 of some 13C molecules (4) for hyperpolarized experiments by reducing the effect of 1H coupling, the focus has mostly been on species with 1H directly bound to the 13C atoms, and little work has been done in the setting of 13C pyruvate (5-6). In addition to the 1H coupling between the 13C atom and the protons on the molecule, there is also coupling to the protons exchanging between the substrate molecule and the aqueous solution7. In this study, we investigated the impact of deuterium enrichment on the T1 of [2-13C]pyruvate, as well as the additional effect from using D2O as the dissolution medium for hyperpolarized imaging.Methods:
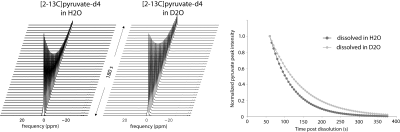
Hardware and hyperpolarized sample: All studies were performed using a 3T GE MR750 scanner (GE Healthcare) with a dual-tuned 1H/13C birdcage rat coil. A SPINLab polarizer (GE) was used to polarize the substrate. Neat [2-13C] pyruvic acid-d4 (Isotec) was doped with 15mM of AH111501 radical (GE). The samples were polarized at a field of 5.0 T and 0.8 K and using the optimal MW frequency (identical to that of [1-13C]pyruvic acid). The dissolution medium was either H2O or D2O (with 100mg / L EDTA) and the dissolved sample solution was neutralized with a NaOH/TRIS buffer post dissolution (also made with either H2O or D2O). MRS Experiments: To estimate the polarization and T1 of hyperpolarized [2-13C]pyruvate-d4 in solution, 13C spectra were acquired from a ~5 ml aliquot of the hyperpolarized solution (H2O: n=2, D2O: n=2) using a pulse-acquire pulse sequence (5 degree flip angle, 5s TR) starting at 60s after the start of the dissolution (10s dissolution time, 20s to transfer sample from polarizer to coil, 30s wait time in the coil). Thermal equilibrium spectra were also acquired.Results and Discussion:
Representative time resolved spectra and signal decay curves from hyperpolarized [2-13C]pyruvate-d4 samples dissolved in H2O and D2O are shown in Figure 1. The signal decay of the pyruvate resonance was noticeably slower with the sample in D2O as compared to H2O. The averaged T1 and polarization measured in solution are summarized in Table 1. With deuterium enrichment on the pyruvate molecule, the T1 of [2-13C]pyruvate increased by 12 s when the sample was dissolved using H2O. When the deuterated pyruvate was dissolved using D2O, the T1 increased by an additional 28 s, a 46% increase compared to the same sample dissolved in H2O. Note that when the estimated polarizations were extrapolated back to the end of dissolution (t = 10 s) using the measured T1, the polarization achieved in solution was similar for samples dissolved with either H2O or D2O. However, at the start of the data acquisition (t = 60 s), the polarization was a factor 1.35 higher for the sample dissolved in D2O, presumably due to the longer T1 and thus slower polarization decay for those samples during sample transfer and wait time.Conclusions:
A modest increase in T1 was observed from hyperpolarized [2-13C]pyruvate when the molecule was enriched with deuterium. A more significant increase in T1 was demonstrated when the sample was dissolved and neutralized using media prepared with D2O, resulting in a net increase of >80% for [2-13C,U-2H3]pyruvate dissolved in D2O. The longer T1 can help preserve the non-equilibrium hyperpolarization during sample QC and transfer, which currently require a significant amount of time (typically 60-90s) relative to T1, and leads to higher polarization at the time of injection.Acknowledgements
This study was supported by the following: CIHR-PJT152928 and NIH/NCI Cancer Center Support Grant P30 CA008748.References
1. Schroeder MA, Atherton HJ, Ball DR, Cole MA, Heather LC, Griffin JL, Clarke K, Radda GK, Tyler DJ. FASEB J. 2009; 23:2529–25382.
2. Chen AP, Hurd RE, Schroeder MA, Lau AZ, Gu YP, Lam WW, Barry J, Tropp J, and Cunningham CH. NMR Biomed. 2012; 25:305–3113.
3. Chung BT, Chen, HY, Gordon J, Mammoli D, Sriram R, Autry AW, Le Page LM, Chaumeil MM, Shin P, Slater J, Tan CT, Suszczynski C, Chang S, Li Y, Bok RA, Ronen SM, Larson PEZ, Kurhanewicz J, Vigneron DB. J Magn Reson. 2019;309:106617.
4. Keshari KR and Wilson DM. Chem. Soc. Rev. 2014;43(5):1627-59.
5. Barb AW, Hekmatyar SK, Glushka JN, and Prestegard JH. J Magn Reson. 2013;228:59–65.
6. Kennedy BWC, Kettunen MI, Hu D-E, and Brindle, K. M. JACS. 2012;134:4969–4977.
7. Cho A, Eskandari R, Miloushev VZ, and Keshari, KR. J Magn Reson. 2018;295:57–62.