3802
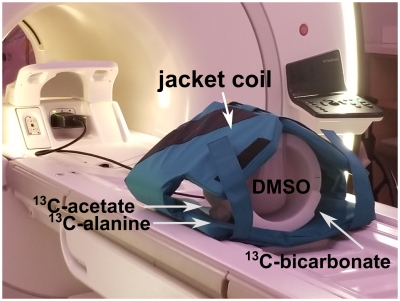
Hyperpolarized 13C Imaging Using a Custom-built 13C Jacket Coil in a 3 T GE Scanner1Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2GE Healthcare, New York, NY, United States, 3Weill Cornell Graduate School, New York, NY, United States
Synopsis
Hyperpolarized MRI using [1-13C] pyruvate is a novel technique that has been safely performed in humans for cancer studies. Here, we present preliminary work on acquiring dynamics of hyperpolarized [1-13C] pyruvate images using a custom-built 13C jacket coil, demonstrating the applicability of the method to image patients with metastatic cancer in the abdomen. The jacket coil provides coverage and RF excitation over the whole abdominal area, allowing to detect the spread of the metastatic cancer. The hyperpolarized 13C images acquired by a spiral sequence show a good quality, demonstrating the feasibility of the method for future hyperpolarization studies in patients.
INTRODUCTION
Abdominal MRI plays an important role in clinical diagnostics, allowing evaluation of abdominal pathology for patients diagnosed with metastatic cancer in the abdomen. Yet, one of the challenges of MRI that limits its widespread applicability is the inherently low sensitivity of magnetic resonance at thermal equilibrium. Hyperpolarization by dissolution dynamic nuclear polarization is an emerging technique that significantly improves the signal to noise ratio by several orders of magnitude. This technique has been successfully developed in conjunction with MRI to image metabolism in humans by administering hyperpolarized metabolic molecules. Here, we show dynamics of hyperpolarized [1-13C] pyruvate images acquired by a custom-built 13C jacket coil, demonstrating its applicability to image patients diagnosed with metastatic cancer in the abdomen for future hyperpolarization studies.METHODS
Hyperpolarization of [1-13C] pyruvateThe 80 μL pyruvate prep consisted of 14.2 M [1-13C] pyruvate and 15 mM free radical (GE healthcare). The prep was hyperpolarized in a 5.0 T SpinLab Hyperpolarizer (GE healthcare). After 3 hours, the prep was rapidly dissolved with preheated 40 mL Tris buffer (100 mM in H2O supplement with 0.1 mM EDTA, pH 7.4). The resulting solution was immediately diluted in H2O to a final concentration of 4.7 mM and transferred to a jacket coil preinstalled in a 3 T scanner (GE healthcare) for signal acquisition.
13C MRI
All MR data were acquired on a wide-bore 3 T scanner (MR750w, GE healthcare). T1 – weighted images (axial and coronal view) were acquired for anatomical reference using a spin-echo sequence. The jacket coil (Clinical MR Solutions) was custom-designed for 13C hyperpolarization studies. It was used for excitation and detection on 13C. The 13C images were acquired using a spiral sequence,1,2 axial, 32 × 32 cm2 field of view, 1 × 1 cm2 in-plane resolution. For the thermally polarized experiment, the images were acquired with 60 scans, 90o flip angle, 6 s repetition time for each 13C resonance, 10 cm single slice. For the hyperpolarization experiment, the images were acquired with 60 scans, 15o flip angle for hyperpolarized [1-13C] pyruvate and 90o flip angle for thermally polarized 13C-urea (6 M, thermal reference), 10 s repetition time for each 13C resonance, 7 slices 1.5 cm/slice.
RESULTS
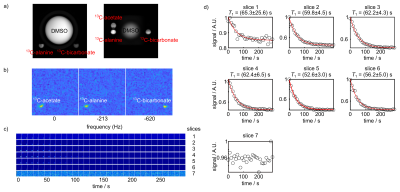
To test the image quality achievable by the jacket coil (Figure 1), T1-weighted images were first acquired to localize the sphere phantoms containing 13C-labelled compounds (Figure 2a). Then single-slice images were acquired on the thermally polarized 13C-labelled phantoms using a spiral sequence. The images of each 13C resonance were generated by summing the signal for each time point (Figure 2b). The locations and signal intensity of the resulting images matched that of the T1-weighted anatomical images and concentrations of the 13C-labelled phantoms, respectively. To further determine the appropriate parameter setup and image quality for future hyperpolarization experiments in patients, dynamics of hyperpolarized [1-13C] pyruvate in a phantom were acquired with the same sequence where multiple slices were excited (Figure 2c). Dynamic curves were generated by summing the signal for each time point within each slice and therefore allowing for obtaining T1 relaxation time of [1-13C] pyruvate (Figure 2d). The derived T1 relaxation time of hyperpolarized [1-13C] pyruvate in H2O is ~ 60 s with a polarization level of 20 %, which will be further improved by using D2O as dissolution solvent.3 The fitted T1 values are consistent among slices which indicates the robustness of the method.DISCUSSION
Hyperpolarized pyruvate imaging have been safely performed in humans for cardiac, brain, and prostate studies.4 These studies investigated cancer metabolism in a region of interest within the body by monitoring the change of the hyperpolarized metabolic flux. However, for patients diagnosed with metastatic cancer, it often requires to image for multiple regions of interest since metastases in cancer spans a wide range of the body and therefore results in lesions in multiple areas. Here we present the preliminary work on acquiring dynamics of hyperpolarized [1-13C] pyruvate by a custom-built 13C jacket coil, with the purpose of demonstrating the applicability of the method to detect the spread of metastatic cancer in the abdomen for future human studies. The jacket coil (Figure 1) provides coverage and 13C RF excitation over the whole abdominal area and therefore allowing to detect lesions in multiple areas. Due to the nonrenewability of hyperpolarized 13C signal, the spiral sequence was used for rapid data acquisition. The sequence allows to excite each 13C metabolite sequentially using spatial-spectral pulses followed by signal read-out by a singlet-shot spiral trajectory, with the prerequisite that the chemical shift frequencies are known beforehand and the magnetic field is homogeneous across the sample. The matched signal to noise ratio of the thermally polarized images with the concentrations of 13C-labelled phantoms as well as the consistency of the fitted T1 values among slices in the hyperpolarization experiment demonstrate the applicability of the method to future human studies.CONCLUSION
In summary, we presented the applicability of using a custom-built 13C jacket coil to image dynamics of hyperpolarized [1-13C] pyruvate. The images acquired by a spiral sequence using the jacket coil exhibit a good quality, demonstrating the feasibility of the method to detect the spread of metastatic cancer for future hyperpolarization studies in humans.Acknowledgements
This work was supported by R01 CA195476, R01 CA237466, R01 CA252037, S10 OD016422, and NIH/NCI Cancer Center Support grant P30 CA008748.References
1. Lau A. Z., Chen, A. P., Ghugre, N. R., Ramanan, V., et al. Rapid Multislice Imaging of Hyperpolarized 13C Pyruvate and Bicarbonate in the Heart. Magn. Reson. Med 2010; 64:1323–1331.
2. Schulte, R. F., Wiesinger, F. Direct Design of 2D RF Pulses Using Matrix Inversion. J. Magn. Reson. 2013; 235: 115–120.
3. Lees, H., Millan, M., Ahamed, F., Eskandari, R. et al. Multi-sample Measurement of Hyperpolarized Pyruvate-to-lactate Flux in Melanoma Cells. NMR Biomed. 2020; e4447.
4. Wang, Z. J., Ohliger, M. A., Larson, P. E. Z., Gordon, J. W., et al. Hyperpolarized 13C MRI: State of the Art and Future Directions. Radiology 2019; 291:273–284.
Figures