3800
Effects of Blood Oxygen Content on Longitudinal Relaxation of Hyperpolarized 13C Agents at 7 Tesla
Keith Michel1, Collin Harlan1, Trevor Mitcham1, Matthew Merritt2, Richard Bouchard1, and James Bankson1
1Imaging Physics, UT MD Anderson Cancer Center, Houston, TX, United States, 2Biochemistry and Molecular Biology, University of Florida, Gainesville, FL, United States
1Imaging Physics, UT MD Anderson Cancer Center, Houston, TX, United States, 2Biochemistry and Molecular Biology, University of Florida, Gainesville, FL, United States
Synopsis
Longitudinal relaxation time constants of hyperpolarized [1-13C]pyruvate, [13C,15N2]urea and [2-13C]dihydroxyacetone in whole bovine blood were measured via low excitation angle dynamic pulse-acquire NMR spectroscopy at 7T. A small lactate signal was produced in scans of pyruvate, corresponding to <1% of the pyruvate precursor signal. All three 13C agents demonstrated shorter T1 relaxation time constants in blood with arterial oxygen content than in blood with venous oxygenation, and the greatest oxygen-accelerated relaxation effect was observed for pyruvate.
Introduction
Dissolution Dynamic Nuclear Polarization (dDNP) permits measurement of in vivo tissue function in ways not possible through established molecular imaging methods.1 The hyperpolarized (HP) agent is typically administered via intravenous injection, and must traverse the vascular tree before interacting with tissues of interest. Due to the highly transient nature of HP signals, the amount of HP agent delivered to capillary blood in these tissues depends in part on the rate of depolarization of the agent in blood prior to the arrival of the bolus in the capillary bed. The apparent longitudinal relaxation rate of the most widely used DNP agent, 13C pyruvate, has been shown to vary with albumin concentration in vitro,2,3 however the effects of blood oxygenation on agent depolarization has not been thoroughly studied. In this work, we investigate the effects of variations in blood oxygenation on the apparent T1 of HP agents that probe cellular metabolism and tissue perfusion.Materials and Methods
Separate quantities of fresh heparinized bovine blood (Animal Technologies, Tyler, TX, USA) were gently bubbled with oxygen and nitrogen gas to enrich oxy- and deoxyhemoglobin content. Aliquots of oxygenated blood were used to reproduce the arterial oxygen state, and mixtures of oxygenated and deoxygenated blood were used to simulate venous blood. Each blood sample was assayed using a spectrophotometric CO-oximeter (GEM OPL; Instrumentation Laboratory, Bedford, MA, USA). All arterial samples had hemoglobin oxygen saturation (sO2) values >95%, and venous samples had sO2 values between 65% and 70%.1 mL of each blood sample was placed in a 10mm Shigemi NMR tube and inserted in a 7T Bruker NMR system equipped with a 10mm broadband probe maintained at a temperature of 37 C. Shim preset values measured in a sample containing 1 mL of blood and 100 µL of D2O were used. HP samples of [1-13C]pyruvate,4 [13C,15N2]urea,5 and [2-13C]dihydroxyacetone6 were prepared in a HyperSense dDNP system using published methods. 100 µL of each HP dissolution was injected into a blood sample and a pulse-acquire 13C spectroscopy scan (TR = 2s, 10 degree excitation angle) was initiated. The primary signal in each set of dynamic HP spectra was integrated across a frequency range corresponding to the full-width at half-max (FWHM) measured in a time-averaged spectrum, and integral values were corrected for the applied excitations by multiplying the spectral integral from the $$$n$$$th repetition by $$$\sec(10^{\circ})^{n-1}$$$. Exponential decay curves of the form $$$y(t) = C + A e^{-t / T_1}$$$ were then fit to these data, and decay time constants were compared for HP agents assayed in blood with arterial and venous oxygen content using a two-sample t-test. All data processing was completed in MATLAB R2020b (The Mathworks, Natick, MA, USA) with custom-written scripts.
Results
Representative time-averaged spectra for each HP agent are shown in Figure 1. No chemical conversion of urea and dihydroxyacetone was observed, however some lactate production was seen in whole blood following administration of pyruvate. The ratio of lactate to pyruvate FWHM integrals in time-averaged spectra was <1% in all blood samples, and no difference in lactate production was observed with blood oxygenation.Blood oxygenation had a statistically significant effect on the measured T1 values for all three HP agents (Figure 2, Table 1), with more rapid depolarization occurring in whole blood with arterial sO2. Relative to the T1 values measured in venous blood, relaxation time constants measured in arterial blood were on average decreased by 23% for [1-13C]pyruvate, 17% for [13C,15N2]urea and 7% for [2-13C]dihydroxyacetone.
Discussion
Our experiments demonstrate that blood oxygenation imparts variations in depolarization rate for HP 13C agents. This effect was strongest for the HP signal corresponding to the carboxyl carbon of pyruvate, but was also observed for carbamide and ketone moieties. While our methods cannot identify the precise mechanism of oxygen-accelerated relaxation, these results suggest that dipole-dipole interactions between the HP 13C nucleus and paramagnetic O2 are a significant factor at 7T, and that the paramagnetic effect of deoxyhemoglobin is less impactful on longitudinal relaxation than that of O2 in whole blood.The observation of lactate production from HP pyruvate in whole blood is notable since it could complicate quantitative analysis of HP imaging data. However, the amounts of lactate observed in these experiments are likely insignificant relative to those typically measured in metabolically active tissues.
Future work will investigate these effects in human blood at MRI field strengths more commonly used for HP imaging in patients. These variations in T1 relaxation with blood oxygenation state could bias quantitative results of HP imaging studies in patients and animals with abnormal sO2, for example due to impairment of respiratory function.
Acknowledgements
This work was supported in part by the National Cancer Institute and National Institute of Diabetes and Digestive and Kidney diseases of the National Institutes of Health (R01-DK105346, R01-CA211150, P30-CA016672). The content is solely the responsibility of the authors and does not necessarily represent the official views of any funding agency.References
- Kurhanewicz J, et al. Analysis of cancer metabolism by imaging hyperpolarized nuclei: prospects for translation to clinical research. Neoplasia. 2011;13(2):81-97.
- Moreno KX, et al. Competition of pyruvate with physiological substrates for oxidation by the heart: implications for studies with hyperpolarized [1-13C]pyruvate. Am J Physiol Heart Circ Physiol. 2010;298(5):H1556-H1564.
- Marco-Rius I, et al. Hyperpolarized singlet lifetimes of pyruvate in human blood and in the mouse. NMR Biomed. 2013;26(12):1696-1704.
- Bankson JA, et al. Kinetic modeling and constrained reconstruction of hyperpolarized [1-13C]pyruvate offers improved metabolic imaging of tumors. Cancer Res. 2015;75(22):4708-4715.
- Reed GD, et al. High resolution 13C MRI with hyperpolarized urea: In vivo T2 mapping and 15N labeling effects. IEEE Trans Med Imaging. 2014;33(2):362-371.
- Moreno KX, et al. Real-time detection of hepatic gluconeogenic and glycogenolytic states using hyperpolarized [2-13C]dihydroxyacetone. J Biol Chem. 2014;289(52):35859-35867.
Figures
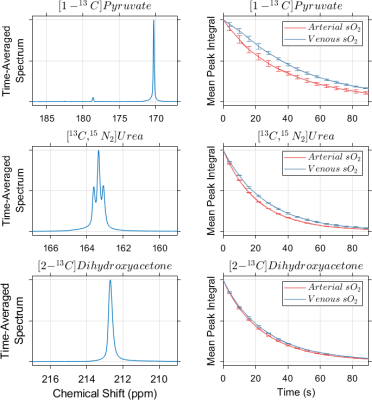
Figure 1. Representative time-averaged 13C spectra measured in
whole blood with venous oxygen content (left) demonstrate minimal chemical
conversion of the HP agents. More rapid longitudinal relaxation of HP signals
was observed in blood with arterial sO2 for all agents tested (right). Error
bars represent one standard deviation about the normalized mean integral
values, and for clarity are plotted at every third timepoint.
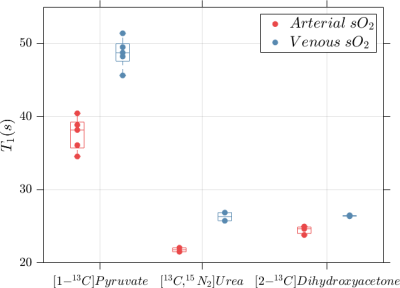
Figure 2. Significantly shorter 13C spin-lattice relaxation time constants were measured for HP agents diluted in blood with arterial oxygenation (>95% sO2) than in blood with venous oxygenation (65-70% sO2).
Table 1. Increased oxygen content in whole blood corresponds to statistically
significant shortening of the apparent 13C T1 relaxation
time constant for all three HP agents tested. P-values were calculated with a
two-sample t-test.