3799
The effect of 2-deoxyglucose and lactate on cell metabolism in acute myeloblastic leukemia ML-1 Cells1The MR Research Centre, Department of Clinical Medicine, The Faculty of Health Sciences, Aarhus University, Aarhus, Denmark
Synopsis
Deoxy-D-glucose (2-DG) is a synthetic glucose analog, which inhibits the first step in the glycolysis by inhibiting the enzyme hexokinase. It has been shown to increase chemosensitivity in some cancers and to reverse glucocorticoid resistance in acute lymphoblastic leukemia. This could have potential for treatment in other leukemia diseases. The present study investigates whether hyperpolarized 13C-MR can be used to explore into the effect of 2-DG and lactate on cell metabolism in acute myeloblastic leukemia cells.
Purpose
One of the hall marks of cancer is metabolic reprogramming where cancer cells switch to anaerobic glycolysis even though oxygen is present. This mechanism is thought to allow cancer cells to master cell growth and cell division because glycolysis provides nucleotides, amino acids, and electron carriers necessary for cell progression [1]. Therefore, targeting cell metabolism therapeutically could be effective in cancer treatment.2-Deoxy-D-glucose (2-DG) is a synthetic glucose analog, which has been shown to inhibit the first step in glycolysis by inhibiting the enzyme hexokinase [2]. 2-DG has been shown to increase radio- and chemosensitivity in breast cancer and to reveres glucocorticoid resistance in acute lymphoblastic leukemia [3, 4]. Therefore, we wanted to investigate how the acute myeloblastic leukemia cell line ML-1 reacted to lactate and 2-DG treatment by measuring the hyperpolarized [1-13C]pyruvate to [1-13C]lactate conversion.Methods and Materials
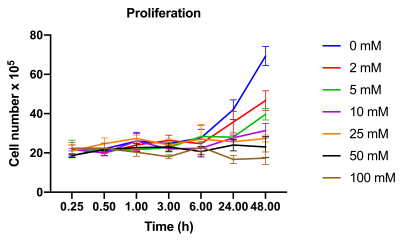
ML-1 cells, a human acute myeloblastic leukemia cell line, were obtained from Sigma-Aldrich. ML-1 cells were cultured in RPMI 1640 (R8758) + 0,3 g/ L-Glutamine + 0,2 g/L glucose supplemented with 10% FBS and 1% penicillin and 1% streptomycin and was maintained in continuous culture at 37 °C in a humidified atmosphere (5% CO2) in an incubator. Growth medium was changed every second or third day.For the viability study a trypan blue exclusion test was used. ML-1 cells were treated with respectively 0, 2, 5, 10, 20, 40 and 100 mM 2 doxy-D-glucose. The cells were counted at 15 m, 30 m, 1 h, 3 h, 6 h, 24 h and 48 h. For all other experiments four groups of cells were prepared, one group of cells was left untreated, one group was treated with 8 mM lactate for 5 minutes, one group was treated with 2 mM 2-deoxy-D-glucose for 24 h and one group was treated with 2 Mm 2-deoxyglucose for 24 h and 8 mM lactate for 5 minutes.Extracellular acidification rate (ECAR) was measured at 37 °C in a closed Unisense-microrespiration double chamber with a lactate-electrode (Unisense A/S, Aarhus, Denmark). For the hyperpolarization experiments [1-13C] pyruvate was polarized in a SpinLab (GE Healthcare, DK) and in a SpinAligner and after injection of 8 mM pyruvate, 13C spectra were continuously acquired over 5 minutes with an interval of 1 second and the lactate-to-all-carbon ratio was calculated. The data was analyzed with the software iNMR (Nucleomatica, Molfetta, Italy).The enzyme activity of PDH and LDH was determined using different assays. The gene expression of HK, LDH and PDH was determined using quantitative PCR and statistical analysis and graphs were made in GraphPad Prism 8.Results
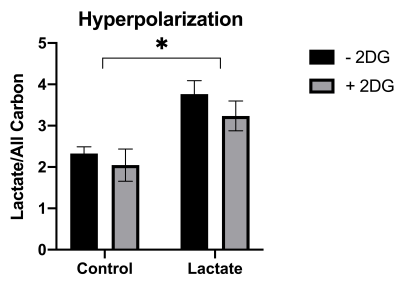
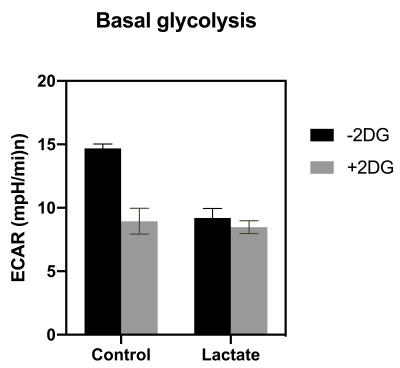
The ML-1 leukemia cells showed significant increase in the lactate-to-all-carbon ratio when lactate was supplemented to the cells compared to the control group. 2-DG treatment of leukemia cells showed a tendency towards a decreased [1-13C]pyruvate to [1-13C]lactate conversion in the groups treated with 2-DG for 24 h compared to the group not treated, indicating that 2-DG inhibits anaerobic glycolysis. 2-DG inhibited cell growth at concentrations > 5 mM after 24 h and at concentrations 2 mM after 48 h and the basal glycolysis in the cells was inhibited by both 2-DG and lactate.Conclusion
2-DG was shown to inhibit cell proliferation, anerobic glycolysis and OXPHOS in ML-1 cells. This could indicate that 2-DG could be used to increase chemosensitivity in AML treatment. Additionally lactate supplement to ML-1 cells instantly increased the [1-13C]pyruvate to [1-13C]lactate conversion, which potentially would be beneficial when using hyperpolarization as a tool to distinguish the metabolic signature between different leukemic cell types.Acknowledgements
We are grateful for the valuable assistance of Duy Anh Dang and Mette Dalsgaard in all aspects of technical handling with the experiments performed at the MR Research Centre, Department of Clinical Medicine, Aarhus University.References
1. Kreitz, J., et al., Metabolic Plasticity of Acute Myeloid Leukemia. Cells, 2019. 8(8).
2. Zhang, D., et al., 2-Deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy.Cancer Letters, 2014. 355(2): p. 176-183.
3. Aghaee, F., J. Pirayesh Islamian, and B. Baradaran, Enhanced radiosensitivity and chemosensitivity of breast cancer cells by 2-deoxy-d-glucose in combination therapy. Journal of breast cancer, 2012. 15(2): p. 141-147.
4. Gu, L., et al., Low dose of 2-deoxy-D-glucose kills acute lymphoblastic leukemia cells and reverses glucocorticoid resistance via N-linked glycosylation inhibition under normoxia. Oncotarget, 2017. 8(19): p. 30978-30991.
Figures