3798
Molecular translation in inhomogeneous field can dramatically reduce the efficiency of spin-order transfer at high field1Department of Radiology, Medical Physics, Medical Center, University of Freiburg, Faculty of Medicine, Freiburg, Germany, 2German Consortium for Cancer Research (DKTK), partner site Freiburg, Freiburg, Germany, 3German Cancer Research Center (DKFZ), Heidelberg, Germany, 4Section Biomedical Imaging, Department of Radiology, University Medical Center Schleswig-Holstein, Campus Kiel, Kiel, Germany
Synopsis
Parahydrogen induced polarization (PHIP) allows providing hyperpolarized (HP) agents for metabolic MRI within seconds and at negligible cost. Recently, we demonstrated PHIP inside of an MRI system using spin-order transfer (SOT) sequences and showed direct in-vivo administration of the agent without transport. Here, we show that molecular translational motion in the inhomogeneous field of the MRI during SOT can strongly corrupt the HP yield. While we already demonstrated signal enhancement of 13C of 40.000-fold at 7 Tesla with our method, we suggest that the HP can be further improved by a better field homogeneity and reduced motion during the SOT.
INTRODUCTION
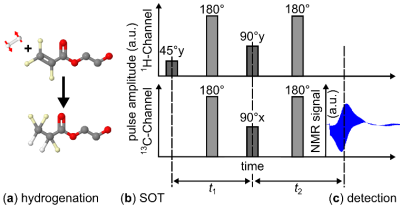
Hyperpolarization (HP) is promising for magnetic resonance (MR), allowing a 104-105-fold increase of the signal. Parahydrogen (pH2) and synthesis allow a dramatically enhanced nuclear alignment (PASADENA)1 is an established method and of great interest due to low cost, fast production and capability to hyperpolarize various metabolites including pyruvate and acetate.2–4 Recently, we demonstrated PASADENA in a 7 T MR imaging system (referred to as SAMBADENA), which allowed administering HP agents in vivo within no more than 5-10s after HP without transport to the MRI setup.5,6 Whereas the theoretically highest HP of 13C of the applied spin-order transfer (SOT) sequence (PH-INEPT+7, Fig. 1) is ~49%, experimentally we achieved up to 25%. We have observed that the injection of pressurized pH2 - that starts the hydrogenation - induces strong inhomogeneities of the static magnetic field (B0) that partially persist after the bubbling and can disturb the SOT.8Here, for the first time, we study the reduction of SOT efficiency due to molecular translation in the reactor in the inhomogeneous field using numerical simulations.
METHODS
For our simulations, we used the product operator and density matrix formalism, assumed instantaneous and perfect RF pulses in the simplified 3-spin system in the laboratory frame. We considered the PH-INEPT+ sequence for the HP of 1-13C hydroxyethyl propionate at 7T; chemical shifts and J-couplings were taken from the literature.9,10First, a “worst-case scenario” was considered: a relative offset of Larmor frequency of each corresponding nucleus, $$$\Omega_0$$$ (created by field inhomogeneities) was applied in both free evolution intervals of the PH-INEPT+ sequence (t1 and t2, Fig.1) such that before and after the refocusing pulses of the sequence the spins evolved at a frequency offset of $$$-\Omega_0$$$ or $$$+\Omega_0$$$ respectively.
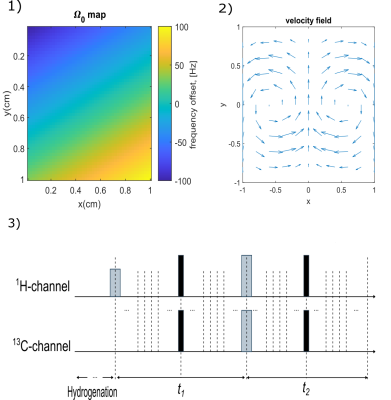
Next, we modeled motion in a 2D plane (a matrix with $$$N_x\times N_y=50\times50$$$) combined with a spatially-varying relative Larmor frequency offset for a non-chemically shifted 1H ($$$\Omega_0$$$). The following simplified conditions were considered:
- A linear change of offset along the two dimensions. The offset along x axis was 2 times stronger than along y to reduce symmetry (Fig. 2.1): $$\Omega_x(x_i)=(\frac{2i}{N_x}-1)\Omega_{0x}, \Omega_y(y_j)=(\frac{2j}{N_y}-1)\Omega_{0y}, i=\{1,...,N_x\}, j=\{1,...,N_y\}.$$
- Time evolution during the pH-INEPT+ sequence was divided into several calculation steps with constant length, dt (Fig. 2.3). The number of calculation steps during t1 and t2 was adjusted, so that dt remained constant. The velocity amplitude of a molecule in the plane, was defined by:
- At each time step, a molecule experienced a single movement in the 2D-plane according to a 2D velocity field (Fig. 2.2):
where $$$L=1$$$cm is the length of the considered plane.
For the simulation, an ensemble of 50 molecules was considered. One after another, they were generated randomly in the 2D plane and subjected to the SOT sequence in the inhomogeneous field. For each magnitude of velocity vm, the final polarization was determined as an ensemble average.
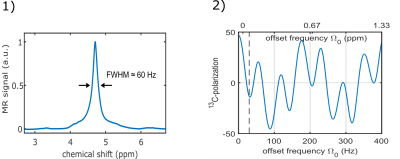
To estimate the field inhomogeneity during the SOT in our experiments, we acquired a 1H spectrum of H2O and determined the full width at half maximum (FWHM) before and after the injection of pH2 (15 bar, 5 s). To this end we used a PRESS sequence localized on the reaction chamber of the reactor used in our experiments.
RESULTS
Fig 3.1 shows the obtained 1H spectrum (FWHM=60Hz).Within the range of the experimentally observed FWHM (i.e. $$$\Omega_0\in[0,30]$$$Hz) a drastic variation of P13C was predicted: $$$P_{13C}=50\%\to-20\%$$$(Fig. 3.2).
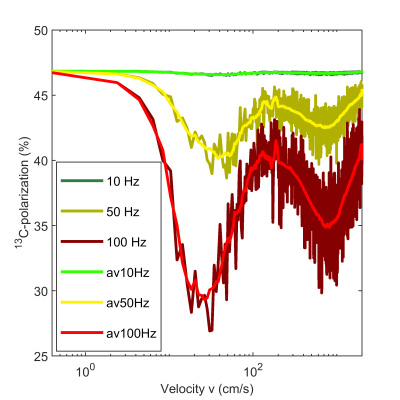
When SOT with motion was modeled for different velocities vm, P13C strongly depended on the magnitude of frequency offset (Fig. 4): for $$$\Omega_0=10$$$Hz translational motion barely effected P13C. However, for larger offsets, $$$\Omega_0=50$$$ and 100Hz, HP significantly decreased from P13C (v=0.4cm/s) to ≈40 and 30%, respectively, at v≈20cm/s. For higher velocities, an increase of P13C was found, although a second minima was detected at ≈700cm/s.
DISCUSSION
In the considered model, prominent loss of SOT efficiency is predicted for velocities v≈10cm/s, when offsets in a range of 50 or 100Hz were considered. At the same time a FWHM of the H2O signal after bubbling of 60Hz was measured, suggesting that our current SAMBADENA implementation may significantly suffer from the effects studied here. However, a solution to this problem will be to achieve a better B0 homogeneity during SOT or adjusting the velocity of the motion.We anticipate a more realistic consideration in the future studies: (a)simulation in a 3D-volume mimicking the shape of the reactor; (b)using measured B0 maps; (c)using a more realistic velocity field, adapted from slow-motion video of the pH2 injection.
CONCLUSION
The results of the simulation show that field inhomogeneities play a crucial role in the performance of SOT sequences at high fields. However, finding technical solutions is of great interest as high-field SOT recently achieved remarkable P13C of esters of the metabolites acetate ($$$P_{13C}\approx60\%$$$) and pyruvate ($$$P_{13C}\approx20\%$$$).3,11 Thereby, HP metabolites could be provided much more cost efficient, within seconds and inside the magnet bore for future metabolic MRI with SAMBADENA.Acknowledgements
ABS and SB want to acknowledge funding support of the Research Commission of the University Medical Center Freiburg (SCHM2146-20) and the German Consortium for Translational Cancer Research (DKTK). ABS further acknowledges support by the Heinrich-Böll foundation (P131623), the Emmy Noether Program of the German Research Foundation (DFG, HO 4604/2‐1, 4604/2‐2) and the Wayne State University (Faculty Postdoctoral Award together with Prof. Eduard Chekmenev, Detroit, US; 177805).References
1. Bowers, C. R. & Weitekamp, D. P. Parahydrogen and synthesis allow dramatically enhanced nuclear alignment. J. Am. Chem. Soc. 109, 5541–5542 (1987).
2. Reineri, F., Boi, T. & Aime, S. ParaHydrogen Induced Polarization of 13 C carboxylate resonance in acetate and pyruvate. Nat. Commun. 6, 5858 (2015).
3. Korchak, S., Mamone, S. & Glöggler, S. Over 50 % 1H and 13C Polarization for Generating Hyperpolarized Metabolites—A para-Hydrogen Approach. ChemistryOpen 7, 672–676 (2018).
4. Cavallari, E. et al. The 13C hyperpolarized pyruvate generated by ParaHydrogen detects the response of the heart to altered metabolism in real time. Sci. Rep. 8, 8366 (2018).
5. Schmidt, A. B. et al. Liquid-state carbon-13 hyperpolarization generated in an MRI system for fast imaging. Nat. Commun. 8, 14535 (2017).
6. Schmidt, A. et al. In vivo 13C-MRI using SAMBADENA. PLOS ONE 13, e0200141 (2018).
7. Haake, M., Natterer, J. & Bargon, J. Efficient NMR Pulse Sequences to Transfer the Parahydrogen-Induced Polarization to Hetero Nuclei. 4.
8. Berner, S. et al. SAMBADENA Hyperpolarization of 13C-Succinate in an MRI: Singlet-Triplet Mixing Causes Polarization Loss. ChemistryOpen 8, 728–736 (2019).
9. Goldman, M., Jóhannesson, H., Axelsson, O. & Karlsson, M. Design and implementation of 13C hyper polarization from para-hydrogen, for new MRI contrast agents. Comptes Rendus Chim. 9, 357–363 (2006).
10. Berner, S. Parahydrogen hyperpolarized metabolites and spin dynamics at high magnetic field. (2020) doi:10.6094/UNIFR/166544.
11. Korchak, S., Yang, S., Mamone, S. & Glöggler, S. Pulsed Magnetic Resonance to Signal-Enhance Metabolites within Seconds by utilizing para-Hydrogen. ChemistryOpen 7, 344–348 (2018).
12. Anderson, P. W. & Weiss, P. R. Exchange Narrowing in Paramagnetic Resonance. Rev. Mod. Phys. 25, 269–276 (1953).
Figures