3797
3D Multi-slice hyperpolarized 129Xe imaging of the human brain
Vira Grynko1,2, Yurii Shepelytskyi1,2, Tao Li3, Ayman Hassan4,5, Karl Granberg4, and Mitchell S. Albert2,3,5
1Chemistry and Materials Science Program, Lakehead University, Thunder Bay, ON, Canada, 2Thunder Bay Regional Health Research Institute, Thunder Bay, ON, Canada, 3Chemistry Department, Lakehead University, Thunder Bay, ON, Canada, 4Thunder Bay Regional Health Sciences Centre, Thunder Bay, ON, Canada, 5Northern Ontario School of Medicine, Thunder Bay, ON, Canada
1Chemistry and Materials Science Program, Lakehead University, Thunder Bay, ON, Canada, 2Thunder Bay Regional Health Research Institute, Thunder Bay, ON, Canada, 3Chemistry Department, Lakehead University, Thunder Bay, ON, Canada, 4Thunder Bay Regional Health Sciences Centre, Thunder Bay, ON, Canada, 5Northern Ontario School of Medicine, Thunder Bay, ON, Canada
Synopsis
Hyperpolarized (HP) 129Xe magnetic resonance imaging (MRI) of the brain can be used to evaluate cerebral perfusion. Currently, all HP 129Xe brain imaging techniques were only performed for single slice imaging. To further develop clinical applications of functional HP 129Xe brain imaging, multi-slice acquisitions are necessary. In this work, we demonstrate the capability of multi-slice human brain imaging with HP 129Xe using 3D gradient echo imaging with a Cartesian readout. This will allow further progress in various HP 129Xe functional brain imaging applications such as perfusion evaluation and hemodynamic response detection.
Introduction
The possibility of performing brain imaging using hyperpolarized (HP) 129Xe was predicted in 19941; however, the first human brain imaging using HP 129Xe was done in 20152. Further development of HP 129Xe brain imaging revealed its potential as a functional imaging modality for visualizing cerebral perfusion3,4, hemodynamic response detection4, stroke diagnosis5 and Alzheimer’s disease6. Despite the potential advantages of HP 129Xe brain imaging, this technique requires substantial improvements. All HP 129Xe brain imaging modalities up to date have utilized single slice images due to the low concentration of HP 129Xe in the brain. HP 129Xe multi-slice acquisition, however, is required for accurate quantification and localization of brain perfusion and correct functional imaging of brain function.Methods
A commercial polarizer (Xemed LLC, NH, USA) was used to polarize enriched (83%) 129Xe gas up to a ~50% polarization level. HP 129Xe was dispensed into four 1L Tedlar bags. A Philips Achieva 3T clinical MR scanner equipped with a dual-tuned 1H/129Xe head coil (Clinical MR Solution LLC, WI, USA) was used for imaging. Two healthy female volunteer participants (mean age 27 ± 2 years) were recruited for this study. High-resolution 1H T2-weighted (T2W) anatomical scans were acquired using a fast spin echo (FSE) technique for brain localization. Imaging parameters were as follow: TR = 3000ms, TE = 80ms, FA = 90°, FOV = 250x250mm2, acquisition matrix = 256x256, slice thickness = 20mm, number of slices =5, gap = 0mm. Following anatomical localization, the participants inhaled 1 L of the HP 129Xe gas and held their breath for a period of 20s. HP 129Xe 3D multi-slice gradient echo (GRE) MRI with a Cartesian readout was performed in both the axial and sagittal orientations: TR = 6.2ms, TE = 1.45ms, flip angle (FA) = 12.5°, BW = 150 Hz/pixel, FOV = 250x250mm2, acquisition matrix = 32x32, slice thickness = 20mm, number of slices =5. The acquisition started 10 s after the breath-hold initialization. The line between anterior and posterior commissures was set on the border of the second and third slices for axial orientation. The middle line of the central sagittal slice was aligned with the line in between the brain hemispheres. Anatomical 1H images had the same orientation as the HP 129Xe images. The image signal-to-noise ratio (SNR) was calculated in custom-written MATLAB scripts (MATLAB R2016b, MathWorks, Inc, Natick, MA) for each slice as the ratio of the mean signal value in a rectangular region of interest, divided by the standard deviation of noise in a similar region of interest located in the background.Results
In vivo axial and sagittal multi-slice HP 129Xe MR images of the human brain are shown in Figures 1B and 2B, respectively. Figures 1C and 2C show HP 129Xe images superimposed on top of 1H anatomical T2W images. The SNR values for each slice were calculated for both axial and sagittal views (Figure 3). The SNR of the axial slices increased towards the second slice (SNR = 18.53±4.63) and had a further decay in higher slices. On a sagittal view, the highest SNR was at the center of the brain (Figure 2B, Figure 3) and it was almost equal in the symmetrical slices in both hemispheres. 129Xe signal hypointensity was observed from the brain ventricles.Discussion
The main disadvantage of currently developed HP 129Xe brain imaging methods with regards to clinically approved conventional techniques is a single slice 2D acquisition. The lack of signal from 129Xe dissolved in brain tissues is a considerable limitation for multi-slice imaging. In this work, for the first time, we demonstrated multi-slice HP 129Xe images of the human brain. The images were acquired 10s into the breath-hold to maximize HP 129Xe uptake in the brain tissues. The use of a 3D gradient echo pulse sequence resulted in a higher 129Xe signal compared to a 2D acquisition due to the additional phase encoding over the z-axis. In addition, a high polarization level of ~50% contributed to the high HP 129Xe MRI signal. All of these factors allowed us to acquire 3D HP 129Xe images using five brain slices. The second slice demonstrated the highest SNR among all axial slices (Figure 2B and Figure 3). It can be explained by the largest amount of brain tissue in this slice. The gradual SNR decrease of the top slices can be explained by the decrease in the amount of grey matter tissue enclosed by these slices. The symmetrical profile of the SNR dependence in the sagittal orientation (Figure 3) indicates an equal distribution of HP 129Xe in both hemispheres, with the highest values near the arteries, which supply the blood with dissolved 129Xe.Conclusions
Multi-slice 3D MRI images of HP 129Xe dissolved in the human brain were acquired for the first time. This provides opportunities for substantial improvements of existing HP 129Xe imaging applications for cerebral perfusion quantification, hemodynamic response detection, Alzheimer’s disease and stroke diagnosis.Acknowledgements
This research was funded by the Ontario Research Fund (ORF-RE-09-029) and the Northern Ontario Academic Medical Association (A-18-05). The authors would like to thank Martina Agostino for contributing to abstract editingReferences
- Albert MS, Cates GD, Driehuys B, et al. Biological Magnetic Resonance Imaging Using Laser-Polarized 129Xe. Nature. 1994;370(6486):199-201. doi:10.1038/370199a0
- Rao M, Stewart N, Norquay G, Griffiths P, Wild J. Imaging the human brain with dissolved xenon MRI at 1 . 5T. In: Intl. Soc. Mag. Reson. Med 23. ; 2015. doi:10.1002/mrm.25417.
- Rao MR, Stewart NJ, Griffiths PD, Norquay G, Wild JM. Imaging human brain perfusion with inhaled hyperpolarized 129Xe MR imaging. Radiology. 2018;286(2):659-665. doi:10.1148/radiol.2017162881
- Shepelytskyi Y, Hane FT, Grynko V, Li T, Hassan A, Albert MS. Hyperpolarized 129Xe Time-of-Flight MR Imaging of Perfusion and Brain Function. Diagnostics. 2020;10(9):630. doi:10.3390/diagnostics10090630
- Rao MR, Norquay G, Stewart NJ, Hoggard N, Griffiths PD, Wild JM. Assessment of brain perfusion using hyperpolarized 129Xe MRI in a subject with established stroke. J Magn Reson Imaging. 2019;50(3):1002-1004. doi:10.1002/jmri.26686
- Hane F, Li T, Plata J-A, Hassan A, Granberg K, Albert M. Inhaled Xenon Washout as a Biomarker of Alzheimer’s Disease. Diagnostics. 2018;8(2):41. doi:10.3390/diagnostics8020041
Figures
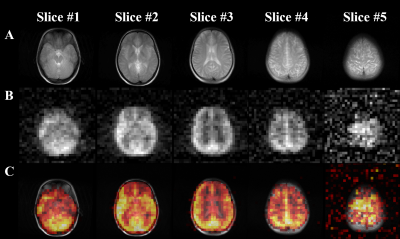
Figure
1. (A) 1H T2W anatomical axial
TSE images of a representative healthy volunteer. (B) HP 129Xe axial brain slice images of a representative
healthy volunteer acquired using 3D GRE imaging 10 s into the breath-hold. (C) HP
129Xe axial brain slice images superimposed on top of the corresponding
1H anatomical images.
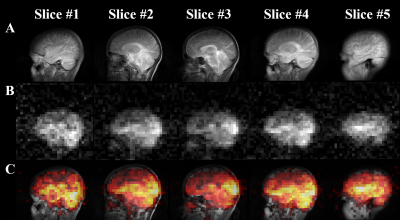
Figure
2. (A) 1H T2W anatomical
sagittal TSE images of a representative healthy volunteer. (B) HP 129Xe sagittal brain slice
images of a representative healthy volunteer acquired using 3D GRE imaging 10 s
into the breath-hold. (C) HP 129Xe sagittal brain slice
images superimposed on top of the corresponding 1H anatomical
images.
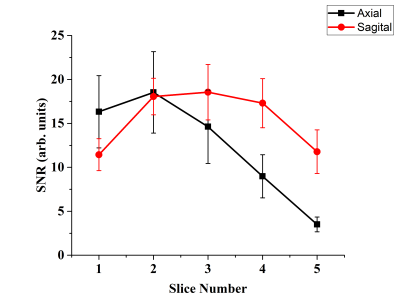
Figure 3. Calculated mean SNR values with standard
deviation in the axial and sagittal orientations for each slice.