3795
Optimized Magnetization Decay Correction of Hyperpolarized 129Xe Ventilation Images Using Radial-Keyhole1Pulmonary Medicine, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 2Biomedical Engineering, University of Cincinnati, Cincinnati, OH, United States, 3Internal Medicine, University of Kansas Medical Center, Kansas City, KS, United States, 4Pediatrics, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States
Synopsis
Advances in hyperpolarization (HP) technology have expanded the translation and clinical utility of HP media MR. Unfortunately, HP images suffer from artifacts and inaccuracies due to magnetization decay. To mitigate decay, we introduced a method to map magnetization dynamics via Bloch-equation modeling and keyhole reconstruction. Here we extend the approach to include optimization via uncertainty propagation. As proof-of-principle, we compared a linear and interleaved keyhole to map HP decay in digital phantoms and 129Xe ventilation images. Linear keyhole yielded uniform decay values, while interleaved keyhole generated physically improbable distributions, demonstrating the utility of analytical optimization in radial-keyhole decay correction.
Introduction
Significant improvements in polarization levels and speed have expanded the utility of in vivo imaging with hyperpolarized media [1,2]. Unfortunately, the initial longitudinal magnetization, Mz(0), is non-equilibrium and decays due to RF and T1 relaxation, introducing image artifacts and complicating quantitative analysis [3, 4]. To correct for HP decay, we introduced a method to map Mz dynamics using Bloch-equation-based modeling and radial keyhole image reconstruction [5, 6, 7]. This method was validated in simulations and phantoms and successfully applied to HP 129Xe ventilation imaging, However, the impact acquisition and reconstruction strategies on image correction have not been rigorously investigated. Here we develop an analytical approach based on uncertainty propagation to optimize radial keyhole correction of hyperpolarized decay and apply it to human lung imaging with HP 129Xe.Background
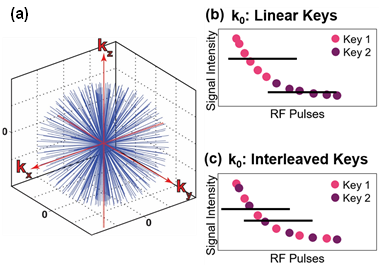
When imaging with center-out trajectories, k0 (i.e. signal at k-space center) is sampled with each acquisition [8] (Fig. 1a), and image intensity is given by the average of k0:(1) $$$S= \frac{1}{N}\sum_{n=1}^NM_z (0) sin(α_{rf}) C_1^{n-1}=\frac{M_z (0)sin(α)}{N} \frac{(1-C_1^N)}{(1-C_1 )} $$$
where (2) $$$ C_1=cos(α) exp(\frac{-TR}{T_1})$$$
and N is the number of views. Because k0 is sampled with each acquisition, the HP decay term, C1, is encoded simultaneously with image data (Fig. 1b, c). This decay is intrinsically low frequency information, so Eq. 1 can be separated into K temporally resolved keys. For example, the data can be separated linearly (ie. key 1 = first half of projections, key 2 = second half) or as interleaves (ie. key 1 = even projections, key 2 = odd projections), yielding the signal intensities given by Eq. 3 and Eq. 4, respectively. C1 can then be extracted for both strategies following Eqs. 5, 6 and decay can be corrected.
(3) $$$S_{κ,L}=M_z (0) sin(α) \frac{C_1^\frac{(κ-1)N}{K}}{N/K}\frac{1-C_1^\frac{N}{K}}{1-C_1}$$$
(4) $$$S_{κ,I}=M_z (0) sin(α) \frac{C_1^\frac{(κ-1)}{K}}{N/K}\frac{1-C_1^N}{1-C_1}$$$
(5) $$$C_{1, L}= ( S_2 /S_1)^{2/N}$$$
(6) $$$C_{1, I}= S_2 /S_1$$$
Image signal can then be corrected—i.e., scaled to yield signal in the absence of magnetization decay, $$$S_0≡M_z (0)sin(α)$$$—by calculating an voxel-by-voxel attention term, A (Eq. 7).
(7) $$$A =\frac{S_0- S}{S_0} = 1-\frac{1}{N}\frac{1-C_1^N}{ 1-C_1}$$$
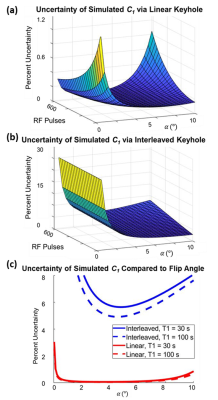
The uncertainty in C1 is dominated by the signal-to-noise ratio, which depends on acquisition parameters (TR, $$$α$$$, etc.) and noise. Error propagation allows this uncertainty to be modeled a priori for the complete range of experimental values and keyhole methods. Uncertainties for linear (Fig. 2a) and interleaved keyhole (Fig. 2b.) are given in Eqs. 8 and 9, respectively.
(8) $$$σ_{C_{1, L} }= σ_S \frac{2}{N}\frac{C_1}{S_1} \sqrt{1+\frac{1}{C_1^N} }$$$
(9) $$$σ_{C_{1, I} }= σ_S \sqrt{(\frac{-S_2}{S_1^2})^2+(\frac{1}{S_1})^2 }$$$
Methods
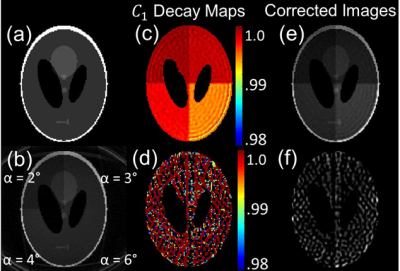
Analytical Simulations: The uncertainty in C1, $$$σ$$$, was simulated as a function of $$$α$$$, T1, and number of RF pulses in MATLAB 2018a.Digital Phantom: A 3D Shepp‐Logan phantom was generated in MATLAB and sampled with 3D radial golden‐angle trajectories, while including HP magnetization decay (Eqs. 1 and 2). Flip angle was varied between 1-10°, T1 = 30 s, N = 410 views, and TR = 5 ms.
Monte Carlo: Digital phantom simulations were repeated with unique Rician noise 30 times to observe C1 variation. The standard deviation of C1 for each parameter space was compared to analytical uncertainty.
In Vivo Imaging: 3D radial MRI was performed with a 3T Philips Achieva and 129Xe polarized to ~40% (Model 9820, Polarean Imaging plc): $$$α$$$ = 1°, TR = 4 ms, TE = 0.118 ms, N =3600 views, BW/pixel = 317.4, FOV = 300 mm.
Results
The predicted uncertainty in signal decay as a function of experimental parameters has a clear minimum (Fig. 2). The appearance of uncertainty minima for both strategies demonstrates that image acquisition parameters directly impact the accuracy of decay correction. Comparison of Fig. 2a and 2b indicates that linear keyhole generates substantially reduced uncertainty.These analytical trends had good agreement with Monte Carlo simulations (e.g., Fig. 3) over a range of flip angles. For T1 = 30 s, TR = 5.3 ms, and N = 410 views, the analytical model predicted a minimum uncertainty at $$$α$$$ = 3.6° and 4.6° for linear and interleaved keyhole, respectively. For Monte Carlo simulation, the minimum occurred at similar values of $$$α$$$ = 3.7° and a = 4.2°, respectively. Further, the interleaved keyhole uncertainty values in the Monte Carlo simulations were 10-fold higher than the linear keyhole approach.
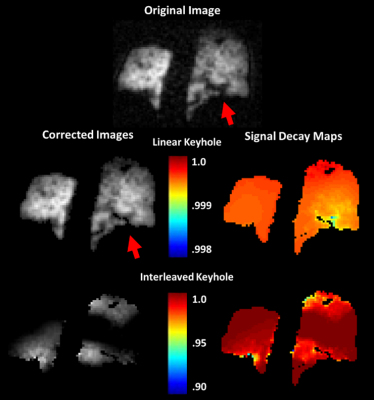
When applied to in vivo imaging data (Fig. 4), the C1 extracted via linear keyhole had low, slowly varying spatial heterogeneity consistent with RF imperfections. In contrast, interleaved keyhole generated physically implausible attenuation values and led to distorted image corrections.
Conclusions
Keyhole correction combined with radial keyhole reconstruction provides a means of correcting HP signal decay without additional data collection. Further, error propagation, when applied to analytical expressions for HP magnetization decay, provides a means to minimize uncertainty, and thus optimize acquisition parameters prior to costly hands-on MRI experiments. Moreover, this analytical approach provides a means of determining the utility of different keyhole reconstruction strategies. Robust corrections were only obtained with the linear method, demonstrating the ability of the analytical approach to meaningfully identify superior keyhole approaches. Because the approach made no assumptions about the nature of the hyperpolarization, this analytical model can be expanded to investigate the impact of reconstruction strategy (e.g., keyhole number) and to other HP nuclei.Acknowledgements
This study was supported by the NIH (NHLBI R01HL143011).References
[1] Walkup LL and Woods JC, NMR Biomed 2014;27(12):1429-38.
[2] Hurd RE, et al., J Magn Resonan Imaging 2012;36(6):1314-28.
[3] Marshal H, et al., NMR Biomed 2012;25(2):389-99.
[4] Miller GW, et al., MAGMA 2004;16(5):218-26.
[5] Niedbalski PJ, et al., Magn Reson Med 2019;82(1): 367-376.
[6] Lethmate R, et al., MAGMA 2003;16(1):21-8.
[7] Subashi E, et al., Med Phys 2013;40:022304.
[8] Glover GH, et al., J Magn Resonan Imaging 1992;2(1):47-52.
Figures