3793
Assessment of Gas Exchange Parameters in Healthy and COPD Subjects using Hyperpolarized Xenon-129 MRI and Alveolar Gas-exchange Model1Ege University, Izmir, Turkey, 2Loughborough University, Loughborough, United Kingdom, 3University of Oxford, Oxford, United Kingdom
Synopsis
Hyperpolarized Xenon-129 MRI ventilation and gas exchange imaging and computational modelling provide a way to quantitatively assess gas ventilation and gas exchange parameters. This new imaging and modelling technique can be used to assess pulmonary diseases including COPD and emphysema and promises to be sensitive to variation in healthy subjects due to age, and/or early phases of pulmonary gas exchange impairment.
Introduction:
Hyperpolarized Xenon-129 (HPX) gas is a respiratory contrast agent in MR for imaging of ventilation defects particularly for chronic obstructive pulmonary disease (COPD) presenting a significant improvement over other lung imaging modalities including Single-photon emission computed tomography (SPECT), and Computed Tomography (CT) (1-3). Although HPX MRI is very sensitive to COPD related ventilation defects, novel HPX MRI techniques also allow the measurement of HPX gas transfer from the lungs into the pulmonary tissue and blood plasma (4, 5). Using appropriate modeling approaches the HPX MRI enables the assessment of gas transfer parameters in addition to ventilation defects (6, 7). In this study, we investigate the utility of a new gas exchange model for quantitative analysis of gas exchange disorders in a COPD cohort in comparison to a healthy cohort.Methods:
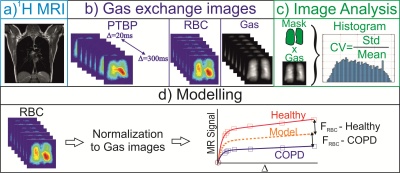
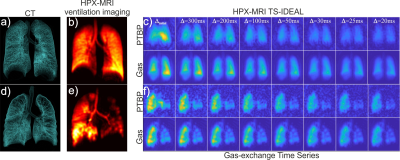
A novel MRI technique based on Iterative Decomposition of water and fat with Echo Asymmetry and Least-square estimation (IDEAL) approach was used to obtain HPX gas-exchange and gas-ventilation images from a total of 18 subjects: COPD (n=9) and healthy subjects (n=9) (5). The COPD subjects were also scanned using high-resolution Computed Tomography (CT). The healthy subjects were scanned using the same HPX gas-exchange MRI protocol. For analysis of image heterogeneity in the ventilation and gas exchange images, the coefficient of variation (CV) was used. Gas exchange curves were obtained as explained in Figure 1. The quantitative analysis of gas exchange curves were performed using a three-dimensional computational fluid dynamic (CFD) model of gas-exchange for the estimation of functional volumes of pulmonary tissue, capillary, and veins. The gas-exchange parameters of the healthy group and COPD were compared using a t-test (Mann-Whitney test, two-tailed, unpaired, and nonparametric).Results:
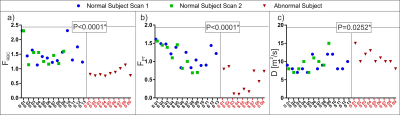
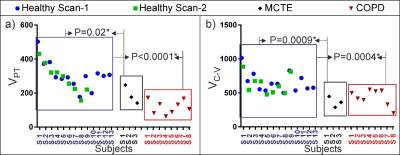
The HPX gas-ventilation and gas-exchange MRI and CT images are shown from a healthy and COPD subject in Figure 2 (a-f). The CV analysis showed that there was a statistically significant difference in gas-transfer and ventilation MRI between the healthy and abnormal cohorts corresponding to the ventilation defects (P<0.0036) in Figure 3. The gas-transfer rate in the COPD group was significantly lower compared to the Healthy group (P<0.001) as shown in Figure 4. Additionally, the functional volume of pulmonary tissue and capillaries were found to be different in the health group (P=0.02*) than minimal CT diagnosed emphysema (MCTE) group whilst the CV measurements were not (P=0.12).Discussion:
In this study, we investigated the measurement of hyperpolarized 129Xe gas ventilation and gas exchange in healthy and COPD subjects using a gas-exchange imaging approach and a gas-exchange model. This new technique enabled the identification of gas exchange variation between the two groups. Additionally, we found that this technique is also sensitive to changes in the gas exchange parameters of a minimal CT diagnosed emphysema group which at present cannot be measured using only gas ventilation imaging.Conclusion:
The novel HPX-MRI gas-exchange and gas-ventilation modeling provides a quantitative assessment of pulmonary gas exchange parameters in healthy and COPD subjects, with the gas-exchange HPX-MRI modeling being more sensitive to variations in pulmonary function due to minimal CT diagnosed emphysema than gas-ventilation HPX-MRI.Acknowledgements
O.D. has been funded by the 2232 International Fellowship for Outstanding Researchers Program of TUBITAK (Project No: 118C189 )References
1. Doganay O, Matin T, Chen M, Kim M, McIntyre A, McGowan DR, et al. Time-series hyperpolarized xenon-129 MRI of lobar lung ventilation of COPD in comparison to V/Q-SPECT/CT and CT. Eur Radiol. 2019;29(8):4058-67.
2. Driehuys B, Martinez-Jimenez S, Cleveland ZI, Metz GM, Beaver DM, Nouls JC, et al. Chronic obstructive pulmonary disease: safety and tolerability of hyperpolarized 129Xe MR imaging in healthy volunteers and patients. Radiology. 2012;262(1):279-89.
3. Qing K, Tustison NJ, Mugler JP, 3rd, Mata JF, Lin Z, Zhao L, et al. Probing Changes in Lung Physiology in COPD Using CT, Perfusion MRI, and Hyperpolarized Xenon-129 MRI. Acad Radiol. 2019;26(3):326-34.
4. Qing K, Mugler JP, 3rd, Altes TA, Jiang Y, Mata JF, Miller GW, et al. Assessment of lung function in asthma and COPD using hyperpolarized 129Xe chemical shift saturation recovery spectroscopy and dissolved-phase MRI. NMR in biomedicine. 2014;27(12):1490-501.
5. Doganay O, Chen M, Matin T, Rigolli M, Phillips JA, McIntyre A, et al. Magnetic resonance imaging of the time course of hyperpolarized (129)Xe gas exchange in the human lungs and heart. Eur Radiol. 2019;29(5):2283-92.
6. Stewart NJ, Parra-Robles J, Wild JM. Finite element modeling of (129)Xe diffusive gas exchange NMR in the human alveoli. Journal of magnetic resonance. 2016;271:21-33.
7. Chang YV, Quirk JD, Ruset IC, Atkinson JJ, Hersman FW, Woods JC. Quantification of human lung structure and physiology using hyperpolarized 129Xe. Magn Reson Med. 2014;71(1):339-44.
Figures