3788
Sensitivity and resolution improvement for in-vivo magnetic resonance current density imaging (MRCDI) of the human brain1Danish Research Centre for Magnetic Resonance, Centre for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital, Amager and Hvidovre, Denmark, 2High-Field Magnetic Resonance Center, Max-Planck-Institute for Biological Cybernetics, Tübingen, Germany, 3Department of Biomedical Magnetic Resonance, University of Tübingen, Tübingen, Germany, 4Section for Magnetic Resonance, DTU Health Tech, Technical University of Denmark, Kgs Lyngby, Denmark, 5Sino-Danish Center for Education and Research, Aarhus, Denmark, 6Department of Neurology, Copenhagen University Hospital, Bispebjerg, Denmark, 7Institute for Clinical Medicine, Faculty of Medical and Health Sciences, University of Copenhagen, Copenhagen, Denmark
Synopsis
Effective use of transcranial electrical stimulation (TES) in clinical and neuroscience applications requires the exact knowledge of TES currents. MRCDI uses MRI to measure the TES-induced magnetic fields for estimating the underlying current flow distributions. Their accuracy highly depends on the sensitivity and spatial resolution of the MR measurements. Here, we propose an advanced gradient-echo-based MRCDI method utilizing an optimized spoiling, acquisition-weighting, and navigators to achieve a noise sensitivity of 84pT at 2×2×3mm3 resolution for a total scan time of less than five minutes. We test the method's performance by phantom and human in-vivo experiments for two TES injection profiles.
Introduction
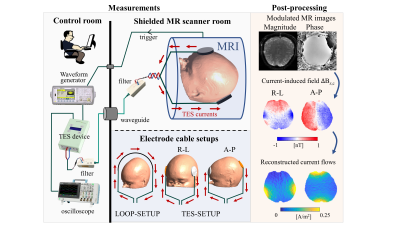
Efficacious use of TES for treating neuropsychiatric disorders or for cognitive enhancement requires the exact knowledge of the current flow induced in the brain. MRCDI uses MRI to measure the magnetic fields created by the TES currents in the head, and reconstructs the current density distribution from these measurements (1). Specifically, the component ∆Bz,c of the TES-induced magnetic field that is parallel to the scanner field modulates the phase of the MR signal. The modulated MR signal can be used for ∆Bz,c calculations and current flow reconstructions (Fig. 1 and 2a) (2). The accuracy of the method highly depends on the sensitivity and spatial resolution of the MRCDI measurements. We have previously reported reliable MRCDI measurements in the human brain with a sensitivity of ~0.1 nT for a ~9 mins scan time (2). Here, we improve our original gradient-echo based MRCDI method by means of an optimized spoiling scheme, acquisition-weighting (AW), and navigators to improve the ∆Bz,c image quality and measurement sensitivity limits at high resolution.Methods
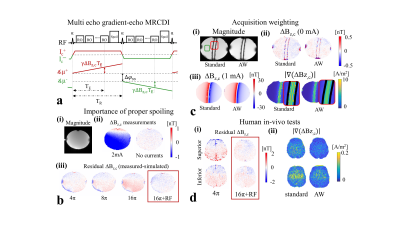
First, we performed MRCDI measurements in a spherical phantom (0.1 mM MnCl2 + 1.5 g/L NaCl) with the LOOP-SETUP in Fig 1 to optimize spoiling: Field of view FOV = 224×183 mm2, imaging matrix 112×92, tip-angle α=30°, echo time TE = [5.6, 14.4, 23.2, 32, 40.8, 49.6] ms, repetition time TR=80 ms, the number of measurement repetitions Nmeas = 16. The experiments with phase-coherent RF excitations were repeated with four different spoiler gradients to provide intra-voxel phase dispersions of $$$\varphi_{sp}=[4π, 8π, 16π,$$$ and $$$32π]$$$. The experiment with $$$\varphi_{sp}=16π$$$ was then repeated with RF spoiling (phase-cycled RF excitations with 50° increments). Second, we employed a spherical phantom (0.1 mM MnCl2 + 1.5 g/L NaCl), in which a cylindrical insulating tube (0.1 mM MnCl2 + 1.5 g/L NaCl + 1 g/L TX151) was placed for current injection, to explore sensitivity gain by means of AW against standard acquisition: FOV = 224×183 mm2, α=30°, TE = [5.6, 14.4, 23.2, 32, 40.8, 49.6, 58.4, 67.2] ms, TR=80 ms, and $$$\varphi_{sp}=16π$$$ combined with RF-spoiling. a) Standard acquisition: Nmeas = 16 was kept fixed for each of the k-space measurements and an image matrix of 112×92 used. b) Acquisition-weighting: The k-space data was acquired in a ~1.6 times broader window and filtered with a Hanning window $$$h(k_x)=\frac{1}{2}(1+cos(\frac{2πk_x∆x}{\beta_w}))$$$ with $$$\beta_w = 1.6$$$ (3). Nmeas was varied systematically to match the applied filter in the phase encoding direction and an imaging matrix of 176×144 was used. We also validated the impact of using optimum spoiling and AW by performing human in-vivo experiments with the LOOP-SETUP using the same method parameters in phantoms. Finally, we recruited five subjects to test our improved method’s human in-vivo performance against our original method using the TES-SETUP. Our original method (2) used Nmeas = 18, TR = 80 ms, TE = [7.5, 19.8, 31.9, 44, 56.2] ms, α=30°, $$$\varphi_{sp}=4π$$$, image matrix 112×90, voxel size 2×2×3mm3 and a chemical-shift-selective fat suppression technique. In our improved method, we acquired extra echoes instead of using fat suppression: TE = [5.6, 14.4, 23.2, 32, 40.8, 49.6, 58.4, 67.2] ms and image matrix 176×144. The first echo was replaced with a 0-D navigator to assess measurement quality. The optimized spoiling scheme ($$$\varphi_{sp}=16π$$$ with RF-spoiling) and AW were applied similar to the phantom experiments. Both experiments used the same FOV = 224×183 mm2 and a matched total scan time of ~4:20 mins for a direct comparison. The experiments were repeated with no currents to measure noise floors and with 1 mA currents injected in the right-left and anterior-posterior directions.Results and discussions
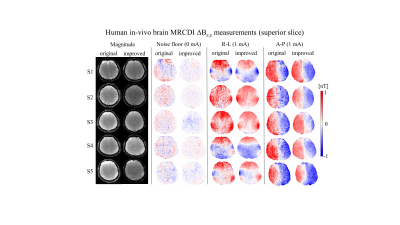
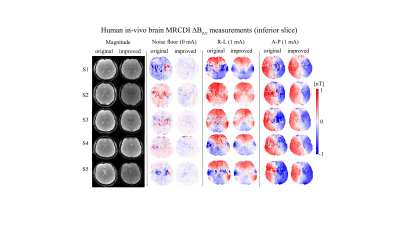
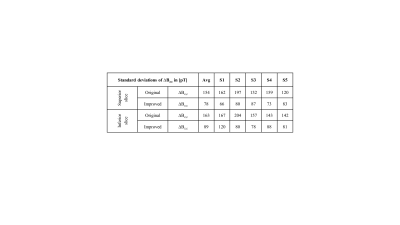
MR magnitude images of the phantoms in Figure 2b,c are artifact-free and the ∆Bz,c spatial distributions are as expected. Interestingly, increasing the spoiler gradients changes the residual ∆Bz,c images (difference between the measured and simulated ∆Bz,c calculated from the delineated cable paths by using the Biot-Savart law (4)). Visual inspection demonstrates two distinct spatial components, one corresponding to the current-induced field and the other to $$$T_2^*$$$. The suggested spoiling scheme ($$$\varphi_{sp}=16π$$$ with RF-spoiling) effectively minimizes the residuals. Using AW significantly improves the quality of ∆Bz,c and its normalized gradient |∇(∆Bz,c)| images (Fig. 2c) and resolves ringing artifacts well. The results translate well to in-vivo (Fig. 2d). Figure 3 and 4 show ∆Bz,c measurements for a superior and an inferior slice. The SNR and image quality greatly improve, and severe artifacts near ventricle regions are resolved by our improved method. On average across the five subjects, the improved method exhibits an almost two-fold sensitivity increase over our original method, with noise standard deviations in the ∆Bz,c images being as low as 78 pT in the superior and 89 pT in the inferior slices for a total scan time of 4:20 mins (Table 1).Conclusion
Our improved method boosted the sensitivity and spatial resolution of MRCDI measurements, and it is thus promising for accurate measurements of TES currents in the brain. Future studies will explore further methodological improvements that can allow for current flow reconstructions in a 3D volume.Acknowledgements
The project is supported by Lundbeck foundation with grant numbers R288-2018-236 and R244-2017-196.References
1. Scott GC, Joy MLG, Armstrong RL, Henkelman RM. Sensitivity of magnetic-resonance current-density imaging. J. Magn. Reson. 1992;97:235–254.
2. Göksu C, Hanson LG, Siebner HR, Ehses P, Scheffler K, Thielscher A. Human In-vivo Brain Magnetic Resonance Current Density Imaging ( MRCDI ). Neuroimage 2018;171:26–39.
3. Budde J, Shajan G, Scheffler K, Pohmann R. Ultra-high resolution imaging of the human brain using acquisition-weighted imaging at 9.4T. Neuroimage 2014;86:592–598.
4. Göksu C, Scheffler K, Siebner HR, Thielscher A, Hanson LG. The stray magnetic fields in Magnetic Resonance Current Density Imaging (MRCDI). Eur. J. Med. Phys. 2019;59:142–150.
Figures