3782
Permittivity Mapping with B1-TRAP1Department of Radiology, University Hospital Basel, Basel, Switzerland, 2Department of Biomedical Engineering, University of Basel, Basel, Switzerland, 3Department of Radiology, Technical University of Munich, Munich, Germany
Synopsis
Permittivity mapping strongly depends on the accuracy and the signal-to-noise ratio (SNR) of the underlying B1+ magnitude estimation method. In this work, we assess the suitability of a transient phase SSFP method, termed B1-TRAP, for permittivity mapping and compare this B1+ mapping method with the commonly-used Actual Flip Imaging (AFI) method.
Introduction
Permittivity mapping strongly depends on the accuracy and the signal-to-noise ratio (SNR) of the underlying B1+ magnitude estimation method. An assessment of different B1+ mapping techniques in the context of permittivity mapping has recently been conducted1. Another promising candidate, termed B1-TRAP (B1+-mapping with TRAnsient Phase SSFP)2, however, has not been investigated so far for its applicability to permittivity mapping. In this work, the B1-TRAP is directly compared to Actual Flip Imaging (AFI) for permittivity mapping in phantom and in vivo.Methods
The quality of AFI and B1-TRAP for permittivity reconstruction was assessed for in vivo human brain and for a spherical water phantom (relative permittivity = 80) doped with 0.125 mM MnCl2 to obtain tissue comparable relaxation times (T1 ~ 870 ms, T2 ~ 70 ms). For AFI, a non-selective excitation pulse with a flip angle of 45° was used with a TR2 / TR1 = 125 ms / 25 ms, a TE = 4.86 ms, a bandwidth of 120 Hz/Px and an RF phase difference increment of 129.3°. An imaging matrix of 88×64×64 was used, yielding an isotropic voxel size of 2.5 mm. The local flip angle was estimated from the signal ratio $$$r$$$ = S2/S1 and the TR ratio $$$n$$$ = TR2/TR1 = 5 using $$$\alpha\approx\arccos\left[\left(rn-1\right)/\left(n-r\right)\right]$$$. In contrast, B1-TRAP derives the local flip angle directly from the transient FID signal oscillations of steady-state free precession (SSFP). For B1-TRAP, a flip angle of 60° was used for the acquisition of a train of 24 echoes with a bandwidth of 250 Hz/px. In contrast to the AFI, which was acquired in 3D, B1-TRAP acquired 31 interleaved slices (with 100% slice distance) with 2.5 mm in plane resolution and 2.5 mm slice thickness within a TR of 5 s. Two scans were performed, resulting in a total of 62 slices and a resolution similar to the AFI method. The local flip angle was estimated using a dictionary. To this end, the B1-TRAP signal was simulated using the configuration model toolkit3 as a function of the flip angle and over a range of T1 and T2 relaxation times. Overall, B1+ mapping with AFI took about 14 minutes and about 11 minutes for B1-TRAP. All scans were carried out on a 3 T clinical MR system (Magnetom Prisma; Siemens Healthcare, Erlangen, Germany) using a 20-channel head coil. Finally, the permittivity was reconstructed from the obtained B1+ maps using4:$$\varepsilon_r\approx-\frac{\nabla^2\left|B_1^+\right|}{\mu\varepsilon_0\omega^2\left| B_1^+\right|}\tag{1}$$
The Laplacian was estimated by local parabola fitting proposed by Katscher et al5 and subsequently smoothed with a tissue boundary–preserving median filter5. The window size for both the Laplace estimation and the median filter was set to 15×15×15 voxels for the phantom and to 21×21×21 voxels for the brain. For AFI, the TR1 magnitude images, and for the B1-TRAP, the inverted T2 map, is used as constraint image for the parabola fitting and boundary-preserving median filtering.
Results
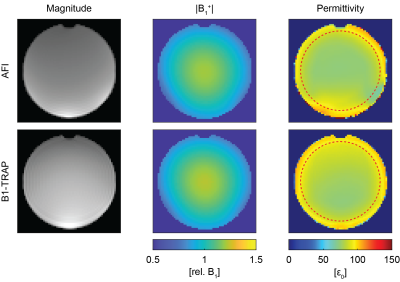
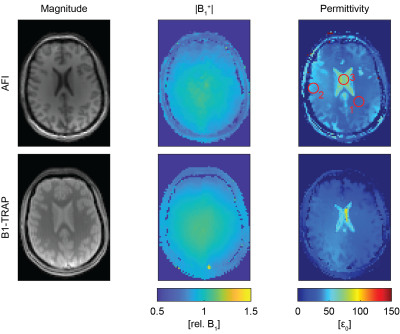
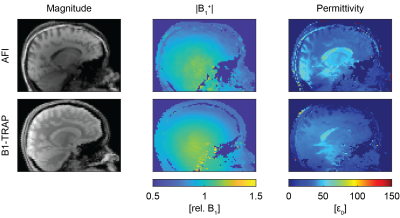
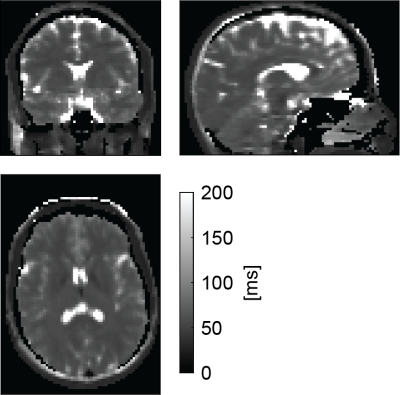
Figure 1 shows for AFI and B1-TRAP an exemplary magnitude slice, together with the corresponding B1+ and the reconstructed permittivity. The AFI permittivity shows a slight inhomogeneity towards the edges of the phantom whereas the permittivity map using B1-TRAP appears more homogenous. For a large region of interest (ROI), relative permittivities of 78.0 ± 4.2 with AFI and 80.6 ± 4.2 with B1-TRAP were found. Figures 2 and 3 show exemplary axial and sagittal images of the in-vivo brain, respectively. Visually, both permittivity maps are of similar quality, but the AFI B1+ map appears to have a higher local variation. Overall, the obtained permittivity values in the brain are underestimated as for relative permittivity values in selected ROIs with AFI yielded 32.2 ± 0.6 for WM, 45.2 ± 1.4 for GM, and 69.5 ± 6.3 for CSF; with B1-TRAP, the values were 32.3 ± 0.4 (WM), 34.7 ± 1.0 (GM), and 62.7 ± 15.6 (CSF). The expected permittivity for WM, GM and CSF are 52, 73 and 84, respectively6. The dictionary allowed simultaneous T2 quantification (see Figure 4), whereas T1 cannot be reliably estimated.Discussion
For B1-TRAP, a 2D rather than a 3D approach was used to mitigate any possible motion sensitivity. As a result, a dictionary was used to account for slice profile effects. For the phantom, B1-TRAP yielded improved permittivity homogeneity. For the in-vivo brain, however, although B1-TRAP visually offered increased signal-to-noise in the B1+ map, due to the large Laplacian kernel used, both methods yield similar results and the permittivity is generally underestimated for AFI as well as for B1-TRAP. While B1-TRAP suffers from poor WM/GM differentiation, the higher contrast in the AFI images results in instabilities in the reconstruction that cause areas of noticeable inhomogeneity. In contrast to AFI, B1-TRAP offers the possibility for simultaneous relaxation estimation from the decay of the transient SSFP signal. The decay offered good sensitivity to T2, whereas T1 could not reliably estimated.Conclusion
In conclusion, we showed that B1-TRAP can be used for permittivity mapping in conjunction with T2 quantification. Paired with a noise-robust reconstruction algorithm, B1-TRAP could be a good candidate for clinical permittivity mapping in vivo at 3 T.Acknowledgements
This work was supported by the Swiss National Science Foundation (SNF grant No. 325230_182008).References
1. Gavazzi S, Berg CAT van den, Sbrizzi A, et al. Accuracy and precision of electrical permittivity mapping at 3T: the impact of three mapping techniques. Magn Reson Med. 2019;81:3628–3642
2. Ganter C, Settles M, Dregely I, Santini F, Scheffler K, Bieri O. B1-mapping with the transient phase of unbalanced steady-state free precession. Magn Reson Med. 2013;70:1515–1523
3. Ganter C. Configuration Model. In Proceedings of the 27th Annual Meeting ISMRM: Paris, France; 2018. Abstract no. 5663
4. Katscher U, Kim D-H, Seo JK. Recent Progress and Future Challenges in MR Electric Properties Tomography. Comput Math Method M. 2013;2013
5. Katscher U, Djamshidi K, Voigt T, et al. Estimation of Breast Tumor Conductivity using Parabolic Phase Fitting. In Proceedings of the 20th Annual Meeting ISMRM: Melbourne, Australia; 2012. p. Abstract no. 3482
6. Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys Med Biol. 1996;41:2271–2293
Figures