3779
Evidence for tissue dielectric property differences between neonates and adults: a retrospective study using MR-EPT1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Synopsis
Electromagnetic simulations for RF safety calculation require knowledge of tissue dielectric properties, but these are not well understood for neonates whose tissues have much higher water content than adults. This work retrospectively used MR-EPT on archived raw data obtained from a study on 800+ neonates, to measure tissue conductivity and permittivity at 128MHz. Presented are initial results from 50 subjects. The measured properties are rather noisy at the individual level, but across the whole cohort the median brain conductivity in neonates is approximately 1.8 times greater than in adults, in line with expectations from scaling arguments.
Introduction
Electromagnetic simulations are a crucial method for evaluating safety of RF coils used in MRI. As well as models of the coil and anatomy of the subject, these simulations require knowledge of the dielectric properties of tissue. Typically, tissue dielectric properties are taken from standard databases1; these are usually based on both in vivo and ex vivo measurements of tissue samples2. While there is general agreement over adult dielectric properties, less is known for pediatric subjects, in particular neonates. Water content in neonatal tissues is much greater than in adults, and this is expected to cause differences in dielectric properties. Indeed, differences in water content are also largely responsible for large differences in NMR relaxation times between adults and neonates.Since no direct measurements of neonatal dielectric properties are widely available, current practice is to estimate them using scaling arguments based on measured dielectric properties in animals, and water content from humans3–5. Using such arguments it is possible to predict that average brain conductivity in a neonate would be a factor of 1.7 greater than an adult, while relative permittivity would be a factor of 1.4 times greater, for example6.Some safety evaluations of RF coils for neonates have accounted for these differences6 while others have not7; there is not yet a consensus. However, our results have shown that differences in tissue properties can lead to significant differences in predicted SAR8.In this work we have used an MRI based electrical-properties-tomography (EPT) method to estimate neonatal properties.Methods
Rather than perform a prospective study, which would be challenging given the availability of neonatal subjects for MRI and demands for other imaging on this cohort, we used the ‘Single Acquisition Electrical Property’ (SAEP) mapping method9 which can reconstruct conductivity ($$$\sigma$$$) and permittivity ($$$\epsilon$$$) using data acquired from multiple receiver coils in a single scan. The method was applied retrospectively to a large database of existing neonatal MRI data, collected as part of the developing human connectome project since 2015. All data was acquired on a 3T Philips Achieva scanner, using a dedicated 32-channel neonatal head coil10. Reconstructed anatomical and functional data from over 800 subjects, ranging 26-45 weeks gestational age at scan, is available to download (http://www.developingconnectome.org/) . The SAEP method requires fully sampled images from each receiver coil with a preferably separate reference. This was obtained from the SENSE reference scans acquired from the 32-channel receiver plus body coil reference which were also archived for all subjects. For this preliminary study, data from 50 neonates (mean age at scan 40.2 weeks PMA, range 36.1-43.7) were processed along with an adult and a phantom (water with 0.9% saline and Gadovist to reduce T1) for comparison. The SAEP method was implemented in Matlab 2020. The FSL BET11 tool was used to define a brain mask from a T1w scan, which was then used to mask the dielectric properties measurements to extract brain averages.Results
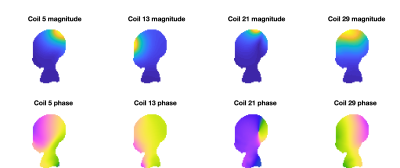
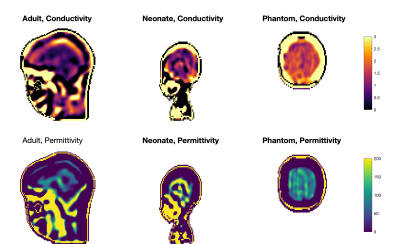
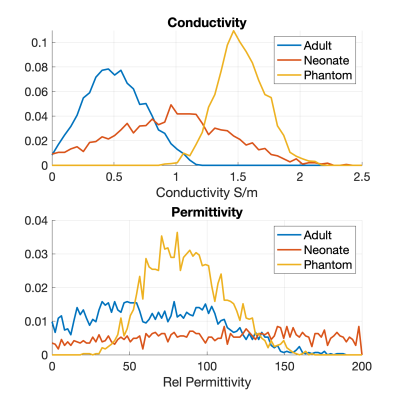
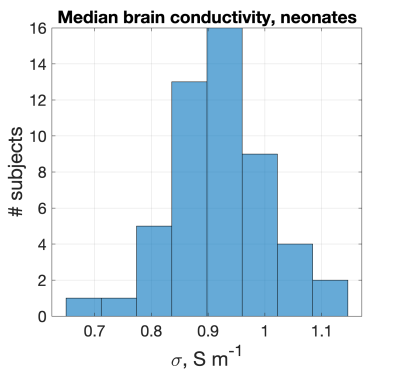
Figure 1 shows example receiver sensitivity maps for one neonate - SAEP requires these images including both amplitude and phase. Figure 2 shows the resulting reconstructed conductivity and permittivity in an adult, neonate, and phantom; Figure 3 shows histograms computed within a sphere of volume 100ml centred in the brain (for the human cases). The estimated conductivities from these histograms are: adult 0.52±0.25Sm-1 (median 0.52), neonate 0.91±0.54Sm-1 (median 0.96), phantom 1.55±0.20Sm-1 (median 1.53). For permittivity the values are: adult 47±56 (median 49), neonate 60±168 (median 90), phantom 88±25 (median 87). Figure 4 shows the distribution of median brain conductivity from a set of 50 neonates obtained from the database. The median brain conductivity is 0.92±0.09Sm-1 across 50 neonates.Discussion
Figure 2 shows that the measured conductivity is noisy but forms a histogram with well-defined peak when averaged over the central brain region-of-interest. The expected adult brain conductivity is 0.47Sm-1 and applying scaling arguments we expected 0.79Sm-1 in neonate6. A 0.9% saline solution at 20°C is expected to have $$$\sigma$$$=1.4Sm-1 12. These expected values are consistent with our measurements. Good permittivity measurements were not obtained in vivo, but the phantom value is in the physically realistic range. The median brain data from 50 neonates (Figure 4) have a significant spread because of noise and some failures of automated brain segmentation, which is yet to be improved. Future work will consider the whole 800+ neonate dataset and look for correlations with gestational age.Conclusion
Results from SAEP on adults and a phantom were in line with expectations. While not sufficiently precise to confirm the calculated values from scaling arguments, the results do support the predictions from scaling arguments based on water content. We find conductivity is greater in neonates than adults by a factor of ~1.8. Hence our results do support the need for age-appropriate dielectric properties. As the authors of the SAEP method also found, this method is better suited for conductivity estimation, and permittivity estimates in vivo are very noisy.In addition to MR safety evaluations these measurements may be useful more widely, for example in other RF modelling studies (telecommunications etc), or for other modalities (EEG, for example).
Acknowledgements
This work was supported by ERC grant agreement no. 319456 (dHCP project), by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and/or the NIHR Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.References
1. Hasgall, P. et al. IT’IS Database for thermal and electromagnetic parameters of biological tissues, Version 4.0, May 15, 2018. doi:10.13099/VIP21000-04-0.
2. Gabriel, C., Gabriel, S. & Corthout, E. The dielectric properties of biological tissues: I. Literature survey. Phys. Med. Biol. 41, 2231 (1996).
3. Seller, M. J. Water content of rat fœtal tissues following administration of water to the dam. Nature 198, 91 (1963).
4. Reinoso, R. F., Telfer, B. A. & Rowland, M. Tissue water content in rats measured by desiccation. J. Pharmacol. Toxicol. Methods 38, 87–92 (1997).
5. Dimbylow, P., Bolch, W. & Lee, C. SAR calculations from 20 MHz to 6 GHz in the University of Florida newborn voxel phantom and their implications for dosimetry. Phys. Med. Biol. 55, 1519 (2010).
6. Malik, S. J. et al. Specific absorption rate in neonates undergoing magnetic resonance procedures at 1.5 T and 3 T. NMR Biomed. 28, 344–352 (2015).
7. Annink, K. V. et al. Introduction of Ultra-High-Field MR Imaging in Infants: Preparations and Feasibility. Am. J. Neuroradiol. 1–6 (2020) doi:10.3174/ajnr.a6702.
8. Malik, S. J., Hand, J. W., Satnarine, R. & Hajnal, J. V. SAR and temperature in neonate models resulting from exposure to 7T head coil. in Proc ISMRM 4616 (2020).
9. Marques, J. P., Sodickson, D. K., Ipek, O., Collins, C. M. & Gruetter, R. Single acquisition electrical property mapping based on relative coil sensitivities: A proof-of-concept demonstration. Magn. Reson. Med. 74, 185–195 (2015).
10. Hughes, E. J. et al. A dedicated neonatal brain imaging system. Magn. Reson. Med. 78, 794–804 (2017).
11. Smith, S. M. Fast Robust Automated Brain Extraction. Hum. Brain Mapp. 17, 143–155 (2002).
12. Stogryn, A. Equations for Calculating the Dielectric Constant of Saline Water (Correspondence). IEEE Trans. Microw. Theory Tech. 19, 733–736 (1971).
Figures