3756
A web-accessible tool for rapid analytical simulations of MR coils via cloud computing
Eros Montin1,2, Giuseppe Carluccio1,2, and Riccardo Lattanzi1,2,3
1Department of Radiology, Bernard and Irene Schwartz Center for Biomedical Imaging, New York University School of Medicine, New York, NY, United States, 2Department of Radiology, Center for Advanced Imaging Innovation and Research (CAI2R), New York University School of Medicine, New York, NY, United States, 3Vilcek Institute of Graduate Biomedical Sciences, New York University School of Medicine, New York, NY, United States
1Department of Radiology, Bernard and Irene Schwartz Center for Biomedical Imaging, New York University School of Medicine, New York, NY, United States, 2Department of Radiology, Center for Advanced Imaging Innovation and Research (CAI2R), New York University School of Medicine, New York, NY, United States, 3Vilcek Institute of Graduate Biomedical Sciences, New York University School of Medicine, New York, NY, United States
Synopsis
DGF is a web-based application to simulate MR coils in the case of simple geometries that mimic actual anatomy. For example, spheres can model the head, whereas cylinders can model the torso, abdomen or extremities. Ultimate intrinsic performance limits can be calculated within the same framework and used as absolute references to evaluate coil designs. DGF relies on rapid analytical electrodynamic simulations based on dyadic Green’s functions, which are executed using Docker containers, either on local computers or via cloud computing. A web-GUI enables users to set up simulations and display results. DGF is part of the Cloud MR project.
Background and Purpose
Analytical electrodynamic simulations have been increasingly used in MRI for rapid modeling of electromagnetic (EM) fields in simple object geometries [1-6]. For example, mode expansions with dyadic Green’s functions (DGF) have been used to express the full-wave EM field in dielectric spheres [7,8] and cylinders [7,9] for simulated MR experiments. Although they use simplified geometries to model anatomical regions, DGF simulations can provide useful physical insights for coil design. For example, they have led to the adoption of electric dipoles for body imaging at ultra-high-field (UHF) MRI. While the theory and equations needed to reproduce previously published results are available in the literature, the practical implementation of a DGF-based computational framework could be challenging and time-consuming. In authors’ knowledge, an open-source implementation of DGF-based simulation software is not available.The purpose of this work was to develop “DGF”, a web-accessible application that enables users to run rapid DGF-based analytical simulations of MR coils via Docker containers, either on local computers or using cloud computing (Figure 1). DGF enables calculating signal-to-noise ratio (SNR), transmit efficiency (TXE) and specific absorption rate (SAR), for both specific coil geometries and the ultimate intrinsic (UI) case, which is independent of any particular coil design. The UISNR, UITXE and UISAR are theoretical limits that enable the prediction of absolute coil performance in simulation [4,8-10], or rigorous performance evaluation of actual coil prototypes [11].Software Architecture and Features
DGF has been developed within the Cloud MR framework (http://cloudmrhub.com, in beta testing, not yet publicly available), which relies on a standardized web-based graphical user interface (GUI) [12,13] to run computational tasks via Docker containers. In particular, the software architecture uses four groups of Docker images that mimic the organization of the human motor system. The “cerebrum” keeps track of all the tasks and read/write information from/in the database, whereas the “brainstem” manages communications with the basic computational units. Each of the latter consist of a “spinal node” component that passes the commands to the “muscles” (one or more), which perform the computations, and send the results back to the brainstem via the spinal node. An additional “cerebellum” component orchestrates the deployment of the computational tasks via containers.As for other applications in Cloud MR [12,13], DGF has a “Home” tab, from which users can manage their data and results files. In the “Set Up” tab (Figure 2), users can customize simulations by choosing the main field strength, as well as the object and coil geometry. In particular, the current implementation of the GUI enables dividing the spherical object into multiple layers, for which users can specify size and dielectric properties (relative permittivity and conductivity) to mimic, for example, different tissues in the human head [14] or evaluate to the effect of integrating high-permittivity materials with coils [15]. Loop coils can be manually positioned or automatically arranged in a symmetric way around the object. In the “Results” tab (Figure 3), users can check the status of the computational tasks and select the successfully completed ones to display the results. Coil performance maps, in terms of SNR (receive coils) and transmit efficiency (transmit coils), as well as transmit and receive field distributions, can be analyzed by means of regions of interest and exported as figures.Discussion and Conclusions
We introduced DGF, a web-accessible application for the simulation of MR coils using analytical calculations based on dyadic Green’s functions. By using analytic calculations, DGF can enable rapid exploration of multiple coil design parameters. DGF can compute coil performance both in parallel receive and parallel transmission. SNR, global SAR and transmit efficiency can be assessed as a percentage of the corresponding ultimate limits. While DGF uses simple geometries to model actual anatomical structures, simulation results can still provide useful physical insight to guide coil design. The current implementation of DGF includes the possibility to model the human head with a multi-layered dielectric sphere. Ongoing work involves the integration of cylindrical geometries to model body imaging applications. DGF is the first open-source software for full-wave analytic simulations of MR coils and it will be made available to the scientific community in 2021 via the Cloud MR portal.Acknowledgements
DGF is available through the Cloud MR project, which is supported in part by NIH R01 EB024536. This work was performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R, www.cai2r.net), a NIBIB Biomedical Technology Resource Center (NIH P41 EB017183).References
- Keltner JR, Carlson JW, Roos MS, Wong ST, Wong TL, Budinger TF. Electromagnetic fields of surface coil in vivo NMR at high frequencies. Magn Reson Med 1991;22:467–480.
- Schnell W, Renz W, Vester M, Ermert H. Ultimate signal-to-noise ratio of surface and body antennas for magnetic resonance imaging. IEEE Trans Antennas Propag 2000;48:418–428.
- Wiesinger F, Boesiger P, Pruessmann KP. Electrodynamics and ultimate SNR in parallel MR imaging. Magn Reson Med 2004;52:376–390
- .Lattanzi R, Sodickson DK, Grant AK, Zhu Y. Electrodynamic constraints on homogeneity and radiofrequency power deposition in multiple coil excitations. Magn Reson Med 2009;61:315–334.
- Carluccio G, Collins CM, Erricolo D. A fast, analytically based method to optimize local transmit efficiency for a transmit array. Magn Reson Med. 2014 Jan;71(1):432-9.
- Vaidya MV, Collins CM, Sodickson DK, Brown R, Wiggins GC,Lattanzi R. Dependence of B1þand B1- field patterns of surface coils on the electrical properties of the sample and the MR operating frequency. Concepts Magn Reson Part B Magn Reson Eng 2016;46:25–40.
- R. Lattanzi,D.K. Sodickson (2012). Ideal current patterns yielding optimal signal-to-noise ratio and specific absorption rate in magnetic resonance imaging: computational methods and physical insights. Magnetic resonance in medicine, 68(1), 286–304. https://doi.org/10.1002/mrm.23198
- Vaidya MV, Sodickson DK, Lattanzi R. Approaching Ultimate Intrinsic SNR in a Uniform Spherical Sample with Finite Arrays of Loop Coils. Concepts Magn Reson Part B Magn Reson Eng. 2014 Aug;44(3):53-65.R.
- Lattanzi, G.C. Wiggins,B., Zhang,Q. Duan,R. Brown, and D.K. Sodickson (2018), Approaching ultimate intrinsic signal‐to‐noise ratio with loop and dipole antennas. Magn. Reson. Med, 79: 1789-1803. https://doi.org/10.1002/mrm.26803
- Georgakis IP, Polimeridis AG, Lattanzi R. A formalism to investigate the optimal transmit efficiency in radiofrequency shimming. NMR Biomed. 2020 Nov;33(11):e4383. doi: 10.1002/nbm.4383.
- Lattanzi R, Grant AK, Polimeni JR, Ohliger MA, Wiggins GC, Wald LL, Sodickson DK. Performance evaluation of a 32-element head array with respect to the ultimate intrinsic SNR. NMR Biomed. 2010 Feb;23(2):142-51. doi: 10.1002/nbm.1435.
- E. Montin, R. Wiggins, K. T. Block, and R. Lattanzi (2019), MR Optimum – A web-based application for signal-to-noise ratio evaluation, 27th Annual Meeting of the ISMRM, 11-16 May 2019, p. 4617.
- E. Montin, G. Carluccio, C. Collins, R. Lattanzi (2020) CAMRIE - Cloud-Accessible MRI Emulator, 28th Annual Meeting of the ISMRM, 08 -14 Aug 2020, p. 1037.
- Bae J, Vaidya M and Lattanzi R, Exploring how modeling the head as a multi-layered vs. uniform sphere affects ultimate intrinsic signal-to-noise ratio and coil performance prediction; 26th Annual Meeting of the ISMRM, 16-21 June 2018, p. 4396.
- Lattanzi R, Vaidya M, Carluccio G, Sodickson DK and Collins CM, Signal-To-Noise Ratio Gain at 3T Using a Thin Layer of High-Permittivity Material Inside Enclosing Receive Arrays, 22nd Annual Meeting of the ISMRM, 11-16 May 2014, p. 4814.
Figures
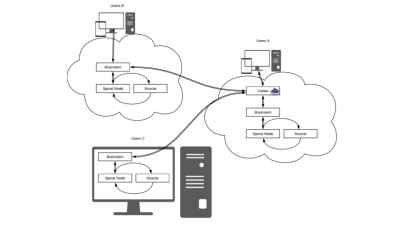
The three types of users supported by DGF. User A requests simulations from the web GUI and runs them on a dedicated Cloud MR AWS account. User B runs the simulations from the web GUI using her/his own cloud computing account. User C runs the simulations on a local computer. Users B and C can synchronize the results on Cloud MR using restful API’s. All types of users can display results on the web GUI or download them in standard formats (e.g., mat or json).
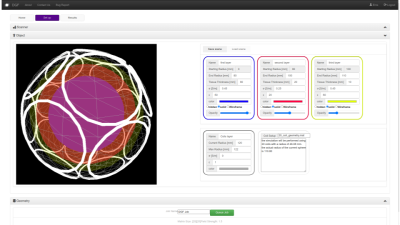
The Set Up tab of DGF’s web GUI. The web GUI is implemented in html and uses several javascripts libraries (angularjs, jquery, bootstrap, plotly.js, math.js and Three.js). The Set Up tab is divided into panels that enable customizing different aspects of the simulations. This figure shows the Object panel, from which users can define the properties of the various object layers (three in this example) and specify the size and position of loop coils.
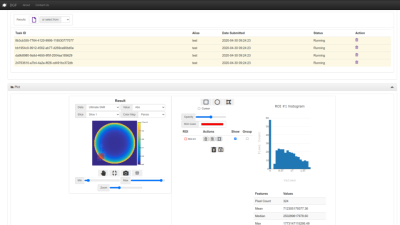
The Results tab of DGF’s web GUI. The Results tab is organized in two panels. From the “Job Queue” panel users can visualize the status of the requested jobs and manage simulation results. A completed job will be highlighted in green, pending jobs in yellow, etc. Users can download the results as json or mat files using restful API’s. The “Plots” panel allows users to visualize simulation results (e.g., coil performance map, coil snr, etc.) and analyze them by drawing regions of interest.