3746
A Multicomponent Image Registration Technique for Largely Deformed Ventricles in Mouse Brain After Stroke1Melbourne Neuropsychiatry Centre, The University of Melbourne, Parkville, Australia, 2The Florey Institute of Neuroscience and Mental Health, The University of Melbourne, Parkville, Australia, 3Department of Biomedical Engineering, The University of Melbourne, Melbourne, Australia, 4Melbourne Brain Centre Imaging Unit, The University of Melbourne, Parkville, Australia, 5Department of Neuroscience, Central Clinical School, Monash University, Melbourne, Australia
Synopsis
A multicomponent registration technique has been proposed to perform image normalization in the presence of largely deformed ventricles in the mouse brain. By employing multicomponent similarity metrics, the proposed technique combines information from multiple filtered and contrast-enhanced copies of the original images in the optimization process. We apply the proposed method to a mouse brain dataset with enlarged ventricles after focal ischaemic stroke, and compare the performance with single-component registration.
Introduction
Animal models of stroke are important to investigate underlying mechanisms of neurodegenerative and neuroprotective processes. Middle cerebral artery occlusion (MCAO) model of stroke results in extensive brain atrophy1,2, including atrophy of the hippocampus2,3,4,5 and ventricle enlargement. MRI experiments offer the potential to investigate long-term structural changes post-stroke. However, MRI image processing methods developed for normal-appearing brain structure show limited performance in stroke images with large-scale deformities.Image registration is a necessary process to spatially normalize images across subjects and time. Mapping individual images to a common reference space enables voxelwise comparisons and facilitates region-of-interest segmentation. Here, we propose a multicomponent non-linear registration (MC-registration) method that utilizes information from multiple filtered and contrast-enhanced copies of original images to optimally map an image to a reference space. We apply MC-registration to an MCAO dataset and quantitively evaluate the performance of MC-registration compared to a single-component technique.
Theory and methods
Multicomponent Non-linear optimizationThe symmetric nonlinear image registration technique (SyN) transforms an image, $$$I$$$, into a reference image space, $$$R$$$, using a two-term optimization process: a shape transformation model and a similarity space6. We propose a multicomponent weighted similarity space, with each component defined by a set of images $$$(I_i,R_i),i\in\{1,...,N\},N$$$ is the number of components in the similarity space and $$$(I_1,R_1)=(I,R)$$$. Each image pair $$$(I_i,R_i),i\ne1$$$ is a filtered and contrast-enhanced copy of original pair. The multicomponent registration (MC-registration) process can be formulated as6:$$\text{arg}\min_v\left(\int_{0}^{1}{\parallel}v(x,t)\parallel_Ldt+\sum_{i=1}^Nw_i\psi_i \right),$$ the first term is the shape transformation model, $$$v(x,t)$$$ is a time-varying velocity-field and $$$L$$$ is an appropriate norm. The second term is a weighted sum of similarity metrics, $$$\psi_i$$$, between $$$(I_i,R_i)$$$.
Animals
All procedures were approved by the Florey Institute of Neuroscience and Mental Health animal ethics committee. Male C57BL/6J mice (n=22) were group-housed in individual ventilation cages under standard laboratory conditions.
Middle cerebral artery occlusion (MCAO)
Transient acute focal cerebral ischemia was induced using the middle cerebral artery occlusion (MCAO) model in 6-week old mice7. Mice were anesthetized with isoflurane. A 6-0 monofilament with silicone-coated tip was inserted via the external carotid artery into the internal carotid artery until a sudden drop in regional cerebral blood flow was observed via the laser Doppler probe. The filament was kept in place for 30 minutes, and then removed for reperfusion. Sham-operated mice were anesthetized, and the carotid arteries exposed without filament insertion.
MRI data acquisition
For MRI, mice were anesthetized with isoflurane and positioned prone on a mouse-cradle with tooth- and ear-bars. Respiration was continuously monitored via a pressure transducer and body temperature was maintained at 35oC using a water circulation system.
MRI was carried out at baseline and 24-weeks post-surgery using a 4.7T MRI with Avance III console and cryogenically cooled transmit/receive surface coil (Bruker, USA). T2-weighted imaging: Rapid Acquisition with Relaxation Enhancement (RARE) sequence, repetition time=12s, TE=40ms, RARE-factor=8, NEX=4, matrix=160x160, FOV=19.2x19.2mm2, 64 slices, slice thickness=0.12mm, resolution=0.12x0.12x0.12mm3.
MRI Analyses
Pre-processing
Images were skull-stripped, bias-field corrected using N4-algorithm8. Mice with ipsilesional hippocampal infarcts and enlarged ventricles were selected for MC-registration (n=5). Ipsilesional ventricle masks were semi-automatically segmented using ITK-SNAP9, and manually corrected. A minimum-deformation template (MDT) was constructed from baseline images using ANTs template construction tool10. A filtered MDT image (MDTfiltered) was obtained using a median filter of size 3x3x3.
Ipsilesional ventricle enhancement
Ipsilesional ventricle enhancement was performed on individual images (Figure 1A). A median filter of size 3x3x3 was applied to each stroke image. Ipsilesional ventricle intensities were upscaled 2.5 times and a ventricle-enhanced filtered image was obtained.
MC-registration
Prior to MC-registration, MDT was affinely registered to individual images (Figure 1B). Three-component SyN optimisation was performed with parameters: r1=8, r2=4 r3=16, w1=0.5 w2=1, w3=1. Component 1 optimized cross-correlation between MDT and individual images, and components 2 and 3 optimized cross-correlation between MDTfiltered and individual images with ventricle enhancement.
Validation
For validation, the ipsilesional ventricles were semi-automatically segmented in MDT and in individual images using ITK-SNAP. Two sets of registration-based ventricle masks were generated, for comparison with manual segmentations. 1) MDT ipsilesional ventricle mask was transformed into individual space using forward transforms from MC-registration (nearest-neighbour interpolation). 2) MDT was registered to original individual images using single-component ANTs registration (sc-ANTs) and ipsilesional ventricle masks were generated using forward transforms. DICE scores between manual and transformed masks were calculated.
Results and discussion
Performance of MC-registrationMC-registration showed improved performance compared to sc-ANTs, with robust neuro-anatomical overlap (Figures 2-3). A slight over-estimation of ipsilesional ventricle-hippocampal boundary was observed.
Quantitative evaluation in individual image space
The dice-index of MC-registration derived ventricle masks (0.60$$$\pm$$$ 0.22) was consistently higher compared to sc-ANTs (0.25$$$\pm$$$0.12) (Table 1). Mouse 2 showed limited registration performance, likely due to enlarged ipsilesional hippocampus and cortex-ventricle deformations (Figure 4).
Quantitative evaluation in template space
The correlation-coefficient between MDT template and MC-registration intensities described stronger correlations in whole-brain (0.82$$$\pm$$$0.03), compared to sc-ANTs images (0.78$$$\pm$$$0.05) (Table 2).
Conclusion
A multicomponent non-linear registration technique has been proposed to perform spatial normalization in the presence of large ventricular deformations in mouse brain after stroke. Future work aims to incorporate hippocampal-specific enhanced components in the proposed method to address overestimation of ventricle-hippocampal boundary.Acknowledgements
This study was supported by an NHMRC Dementia Research Team Grant (DRTG: APP1094974)References
[1] Delattre, C., Bournonville, C., Auger, F., Lopes, R., Delmaire, C., Henon, H., . . . Bastide, M. (2018). Hippocampal Deformations and Entorhinal Cortex Atrophy as an Anatomical Signature of Long-Term Cognitive Impairment: from the MCAO Rat Model to the Stroke Patient. Transl Stroke Res, 9(3), 294-305.
[2] Xie, M., Yi, C., Luo, X., Xu, S., Yu, Z., Tang, Y., . . . Wang, W. (2011). Glial Gap Junctional Communication Involvement in Hippocampal Damage after Middle Cerebral Artery Occlusion. Ann Neurol, 70(1), 121-132.
[3] Firbank, M. J., Burton, E. J., Barber, R., Stephens, S., Kenny, R. A., Ballard, C., . . . O’Brien, J. T. (2007). Medial Temporal Atrophy Rather than White Matter Hyperintensities Predict Cognitive Decline in Stroke Survivors. Neurobiol Aging, 28(11), 1664-1669.
[4] Pohjasvaara, T., Mäntylä, R., Salonen, O., Aronen, H. J., Ylikoski, R., Hietanen, M., . . . Erkinjuntti, T. (2000). MRI Correlates of Dementia after First Clinical Ischemic Stroke. J Neurol Sci, 181(1), 111-117.
[5] Schaapsmeerders, P., van Uden, I. W., Tuladhar, A. M., Maaijwee, N. A., van Dijk, E. J., Rutten-Jacobs, L. C., . . . Kessels, R. P. (2015). Ipsilateral Hippocampal Atrophy is Associated with Long-Term Memory Dysfunction after Ischemic Stroke in Young Adults. Hum Brain Mapp, 36(7), 2432-2442.
[6] Avants, B. B., Epstein, C. L., Grossman, M., & Gee, J. C. (2008). Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Medical image analysis, 12(1), 26-41.
[7] Brait, V. H., Tarrasón, G., Gavaldà, A., Godessart, N., & Planas, A. M. (2016). Selective Sphingosine 1-Phosphate Receptor 1 Agonist Is Protective Against Ischemia/Reperfusion in Mice. Stroke, 47(12), 3053-3056.
[8] Tustison, N. J., Avants, B. B., Cook, P. A., Zheng, Y., Egan, A., Yushkevich, P. A., & Gee, J. C. (2010). N4ITK: improved N3 bias correction. IEEE transactions on medical imaging, 29(6), 1310.
[9] Yushkevich, P. A., Piven, J., Hazlett, H. C., Smith, R. G., Ho, S., Gee, J. C., & Gerig, G. (2006). User-Guided 3D Active Contour Segmentation of Anatomical Structures: Significantly Improved Efficiency and Reliability. Neuroimage, 31(3), 1116-1128.
[10] Avants, B. B., Tustison, N. J., Song, G., Cook, P. A., Klein, A., & Gee, J. C. (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage, 54(3), 2033-2044.
Figures
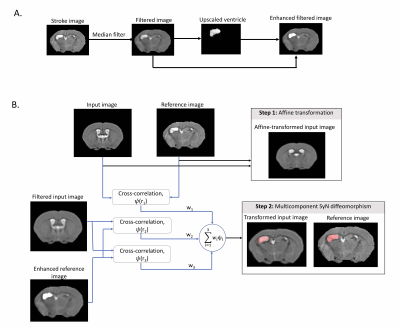
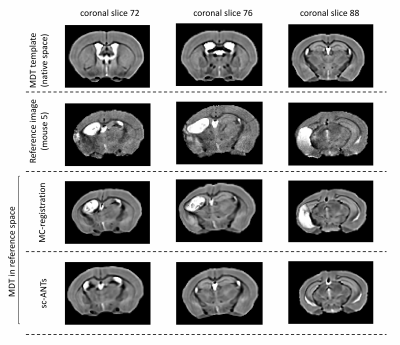
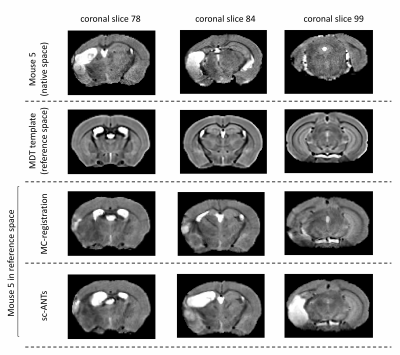
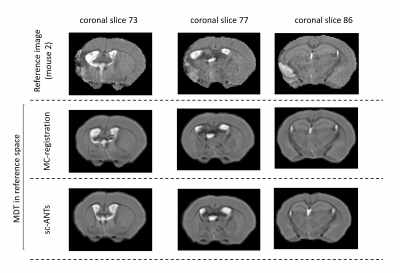
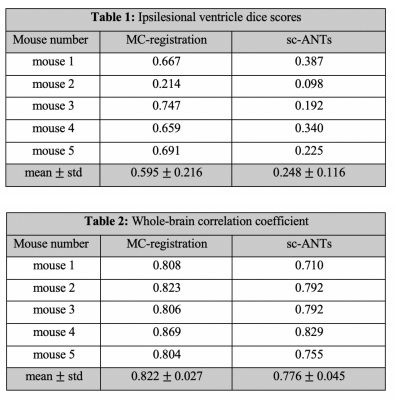
Table 1: Dice scores between reference ipsilesional ventricle masks and masks generated using sc-ANTs and MC-registration. MC-registration outperforms sc-ANTs in all mice. Limited segmentation accuracy in mouse 2 due to hippocampal enlargement.
Table 2: Whole-brain correlation coefficients between MDT (healthy template) and individual mouse brain in MDT space. MC-registration outperforms sc-ANTs in all mice.