3744
Performance evaluation of a Compressed Sensing SWI technique on a clinical 7T MRI system1Radiology, Mayo Clinic, Rochester, MN, United States, 2Siemens Medical Solutions USA, Inc., Rochester, MN, United States, 3Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
A compressed sensing technique was adapted for use in acquiring 3D susceptibility-weighted image data on a clinical 7T MRI system. The image quality was highly dependent on the choice of regularization parameter used in the reconstruction for all accelerated acquisitions, with some residual artifacts manifest in images thought due to the acquisition of anisotropic voxels. Nevertheless, CNR and vein detectability was significantly enhanced in images acquired using the CS-SWI technique compared to the clinical SWI protocol, for CS acceleration factors up to 7.2 (corresponding to a 48% scan time reduction compared to the clinical protocol).
Introduction
Acquisition times for clinical 7T MRI examinations tend to be longer than those at lower field strengths due to SAR-related constraints and the acquisition of higher resolution datasets. Thus, for example, while one can acquire excellent image quality in 2D-TSE acquisitions with 1mm slice thickness, the large number of slices required to cover the brain results in the SAR threshold being exceeded, and requires dividing the acquisition into 2 concatenations, with a resultant doubling of the acquisition time1. Parallel imaging acceleration factors higher than those used at 3T can be used to mitigate the long scan times; this arises from the higher inherent SNR at 7T and the more localized nature of the B1- fields which helps to reduce g-factor noise at higher GRAPPA factors. Nevertheless, there is a clear need to implement faster imaging techniques for 7T MRI to realize its true clinical potential. Compressed sensing approaches are beginning to significantly impact conventional clinical MR imaging particularly in inherently sparse 4D acquisitions (time-resolved MR angiography2 and dynamic contrast-enhanced3 imaging) and more recently for 3D4 and even 2D5 anatomical imaging. However, CS approaches have not yet been adopted for clinical imaging at 7T. A recent study described an evaluation of a CS technique for time-of-flight angiography at 7T, where an acceleration factor of 9.2 was found to produce good image quality in healthy volunteers6. The aim of this study was to adapt this technique for use in acquiring susceptibility-weighted image (SWI) data on a clinical 7T MRI system and evaluate the optimal CS acceleration factor, k-space sampling pattern and regularization parameter in the resultant image reconstruction.Methods
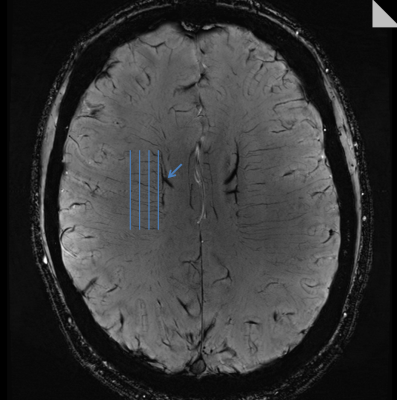
All experiments were performed on a 7T MRI system (MAGNETOM Terra, Siemens Healthcare, Germany) using the regulatory-approved head coil (1Tx/32Rx, Nova Inc.), with approval from the local institutional review board. The CS-SWI technique was based on a 3D GRE sequence, with sparse k-space undersampling implemented via a highly under-sampled variable-density Poisson disk with a Gaussian density distribution2 and was implemented as prototype software. The k-space sampling pattern was varied by defining a Poisson disk radius as a percentage of the diagonal in the phase encoding planes, and acquiring a combination of a fully-sampled 10% k-space center with the remaining samples distributed out to 71%. Acceleration factors of 4.6, 5.5, 6.3, 7.2, 8.8 were investigated, resulting in acquisition times of 4:35, 3:52, 3:21, 2:58, 2:26 minutes, respectively. The CS-SWI datasets were reconstructed using a regularization parameter (RP) value range {0.0001 to 0.01}. At each acceleration factor, an optimal RP value was determined based on the prevalence of image artifacts. The CS-SWI acquisitions were compared to our clinical SWI protocol, with otherwise identical acquisition parameters used throughout (voxel=0.3x0.3x1.2mm3, FOV=220x165x144mm3, TR/TE=22/15ms, flip=15°, rBW=140Hz/px, GRAPPA_factor=3, partial_Fourier=7/8, TA=5min43s). Quantitative analyses were performed via CNR measurements in a large vein (Figure 1), measurements of image differences with the clinical protocol via the structural similarity index (SSI7), and a peak-finding algorithm to count the number of deep medullary veins in the centrum semiovale valley (Figure 1) using custom Matlab code.Results
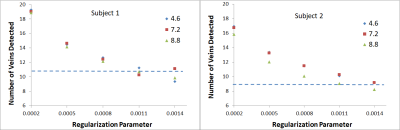
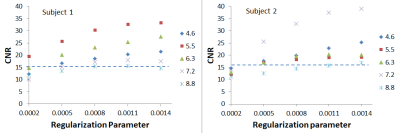
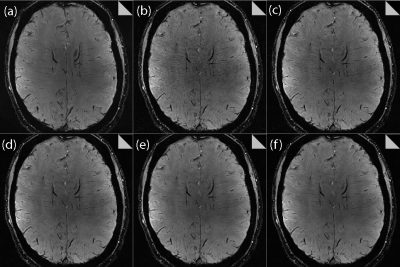
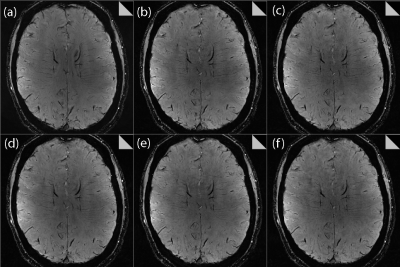
The RP was found to exert a significant impact on the image quality for all CS datasets. As shown in Figure 2 for both volunteers, increasing the RP resulted in fewer veins being detected, as expected from the increased image smoothing caused by higher RP values. However, for all acceleration factors investigated, the CS data outperformed the clinical protocol for number of veins detected for RP values < 0.0011. Increasing the RP was found to increase the measured CNR, as shown in Figure 3; here again, all CS data outperformed the clinical protocol for RP values > 0.0005. Very high/low RP values (0.01/0.0001) produced extremely smooth/noisy images, respectively; Figure 4 shows the effect on image quality of an increasing RP for the factor 4.6 CS acceleration factor dataset for a narrower range of RP values. The effect of an increasing CS acceleration factor on the image quality is shown in Figure 5, where an optimal RP was used to reconstruct the images in each case. SSI analyses revealed correlations in the range 0.45 to 0.47 for the CS acceleration factors with optimized RP values used for the image reconstruction.Discussion
The image quality demonstrated more extreme sensitivity on the choice of RP compared to previous work investigating CS for TOF-MRA5, with widespread, significant image artifacts when the RP was too low, and excessive smoothing effect when too high. Some residual aliasing is evident in all CS-reconstructed images, but nevertheless good overall anatomical visibility is maintained up to an acceleration factor of 7.2. The acquisition of isotropic voxels should reduce residual artifacts. The low SSI values for different RP and acceleration factors may be due to through-plane motion artifacts which were not eliminated.Conclusions
CNR and vein detectability was enhanced in images acquired using the CS-SWI technique compared to the clinical protocol for CS acceleration factors up to 7.2 (corresponding to a 48% scan time reduction). The image quality was highly dependent on the RP value used for the reconstruction for all accelerated acquisitions, with some residual artifacts manifest in images thought to be due to the acquisition of anisotropic voxels.Acknowledgements
No acknowledgement found.References
1. Fagan AJ, Welker KM, Amrami KK, et al. Image Artifact Management for Clinical Magnetic Resonance Imaging on a 7 T Scanner Using Single-Channel Radiofrequency Transmit Mode. Invest Radiol. 2019;54(12):781-791.
2. Stalder AF, Schmidt M, Quick HH, et al. Highly undersampled contrast-enhanced MRA with iterative reconstruction: Integration in a clinical setting. Magnetic Resonance in Medicine. 2015;74(6):1652-1660.
3. Matcuk GR, Jr., Gross JS, Fritz J. Compressed Sensing MRI: Technique and Clinical Applications. Advances in Clinical Radiology. 2020;2:257-271.
4. Fritz J, Raithel E, Thawait GK, Gilson W, Papp DF. Six-Fold Acceleration of High-Spatial Resolution 3D SPACE MRI of the Knee Through Incoherent k-Space Undersampling and Iterative Reconstruction-First Experience. Invest Radiol. 2016;51(6):400-409.
5. Gersing AS, Bodden J, Neumann J, et al. Accelerating anatomical 2D turbo spin echo imaging of the ankle using compressed sensing. Eur J Radiol. 2019;118:277-284.
6. Meixner CR, Liebig P, Speier P, et al. High resolution time-of-flight MR-angiography at 7 T exploiting VERSE saturation, compressed sensing and segmentation. Magnetic Resonance Imaging. 2019;63:193-204.
7. Zhou W, Bovik AC, Sheikh HR, Simoncelli EP. Image quality assessment: from error visibility to structural similarity. IEEE Transactions on Image Processing. 2004;13(4):600-612.
Figures