Subechhya Pradhan1,2,3, Sudeepta Basu4, Kushal Kapse1, Devon Fisher1, Stephanie Norman1, and Catherine Limperopoulos1,2,3
1Developing Brain Institute, Children's National Hospital, Washington, DC, United States, 2Radiology, Pediatrics, George Washinton University, Washington, DC, United States, 3Radiology and Diagnostic Imaging, Children's National Hospital, Washington, DC, United States, 4Neonatalogy, Children's National Hospital, Washington, DC, United States
Synopsis
Preterm brain metabolism studies using magnetic resonance spectroscopy (MRS) demonstrate an association between abnormal biochemistry and brain injury. To our knowledge, there are no reports on the reproducibility of these measurements as well as the optimal pulse sequence to measure each metabolite in the preterm brain. In this study, results of same-session reproducibility of metabolite concentration measurements from 15 preterm scanning sessions using 4 different pulse sequences are presented.
Introduction
Technical advances have allowed in vivo measurements of metabolite concentrations in the preterm brain using MRS. Studies of metabolite trajectories and brain biochemistry in preterm infants can be valuable in expanding our ability to probe the poorly understood mechanisms of development, maldevelopment and increased vulnerability to brain injury (1-3). Optimizing metabolite measurement reproducibility and choice of pulse sequence and parameters is crucial to ensuring reliability of the observed changes in studies; which unfortunately remains largely unexplored in this high-risk population. The objective of this study is to determine which pulse sequence parameters yield the best measurement reproducibility for each metabolite of interest.Methods
We prospectively recruited 13 preterm babies born before 35 weeks of gestational age (GA) from the Children’s National Hospital level IV NICU in accordance with local IRB. Two of the babies were studied twice, yielding a total of 15 scan sessions. All preterm babies in the study were scanned using MR scanner (Discovery MR750; GE Healthcare, Milwaukee, Wisconsin) using a 8-channel head coil (GE Healthcare). Four sets of spectra were acquired using i) PRESS TE/TR: 35ms/2000 ms, ii) PRESS TE/TR: 144ms/2000 ms, iii) STEAM TE/TM/TR: 14/50/2000 ms, and iv) MEGA-PRESS TE/TR: 68/2000 ms with editing frequency at 1.9 and 7.8 ppm on alternate scans. After undergoing frequency and phase alignment of the spectra using programs written in Matlab, metabolite concentrations were quantified in LCModel (4) using water as an internal reference. Metabolites with CRLB >100% were excluded from further analysis. Coefficient of variation (CoV) and absolute difference (AbsDiff) were used as metrics of metabolite measurement reproducibility.Results/Discussion
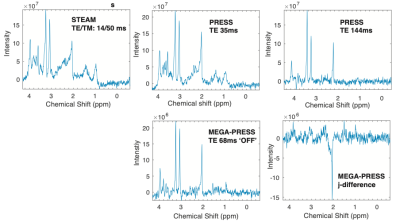
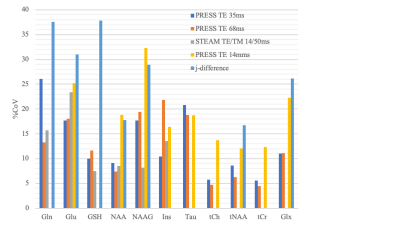
Figure 1 shows LCModel analysis results of typical spectra acquired from the right basal ganglia using different pulse sequences Our results show excellent reproducibility of Choline, Creatine and N-acetylaspartate measurements with CoV<10% for PRESS TE35ms, PRESS TE68ms (OFF acquisitions from MEGA-PRESS) and STEAM TE/TM: 14/50ms and good reproducibility for PRESS TE144ms with CoV<15% (Figure 2). Lactate was consistently detected (>50% of the scans) only using PRESS TE144ms with CoV = 36.2% and AbsDiff = 56.1%. GABA was measured with CoV (%) = 20.5, 23.3 and 25.4 and AbsDiff (%) = 29.0, 32.9 and 36.0 using j-difference and MEGA-PRESS TE 68ms ‘OFF’ acquisition and STEAM TE/TM: 14/50 ms, respectively. CoV for glutamate were: 17.7, 18.0, 18.9, 25.2 and 31.0 using PRESS TE35ms, MEGA-PRESS TE 68ms ‘OFF’ acquisition, STEAM TE/TM 14/50ms, PRESS TE 144 ms and j-difference measurements, respectively. Our results suggests MEGA-PRESS TE=68 ms to be optimal choice for measuring a larger range of metabolites and TE =144 ms for acquisitions where lactate is of main interest.Conclusion
We report that Choline, Creatine, N-acetylaspartate, glutamine, glutamate and GABA concentrations can be reproducibly measured at 3T using 1H MRS. MEGA-PRESS sequence allows reproducible measurement of the largest range of metabolites between ‘OFF’ and ‘j-difference’ acquisition. If the specialized MEGA-PRESS sequence is not available in the clinical setting, PRESS TE 35ms measures a wide range of metabolites with high reproducibility.Acknowledgements
The study was supported by grant funding from National Institutes of Health -Intellectual and Developmental Disabilities Research Center award number 1U54HD090257 and the National Institutes of Health - National Center for Advancing Translational Sciences award number UL1TR001876 and KL2TR001877.References
M. Brossard-Racine et al., Scientific Reports 7, 8143 (2017); S. Tanifuji et al., Brain and Development 39, 196-202 (2017); S. K. Basu et al., Scientific Reports 10, 10549 (2020); S. W. Provencher, Magnetic Resonance in Medicine 30, 672-679 (1993).