3728
Upper brainstem GABA levels in Parkinson’s disease1Shandong Medical Imaging Research Institute, Shandong University, jinan, China, 2Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, baltimore, MD, United States, 3FM Kirby Center for Functional Brain Imaging, Kennedy Krieger Institute, baltimore, MD, United States
Synopsis
The aim of this study is to test the hypothesis that levels of gamma-aminobutyric acid (GABA) in the upper brainstem are reduced in patients with PD compared to healthy controls, using edited magnetic resonance spectroscopy (MRS of GABA+). GABA+-edited MRS was performed in 7.5-ml voxels in the upper brainstem in 18 PD patients and 18 age- and sex-matched healthy controls (HCs), and the spectra were processed using the Gannet software. The lower GABA+ levels in the upper brainstem of the PD patients suggest that a GABAergic deficit in the brainstem may contribute to the dopaminergic pathology in PD.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by dysfunctional motor control resulting from degeneration of dopaminergic neurons in the substantia nigra (SN) [1]. Many circuits involving dopaminergic projections from the midbrain also include GABAergic inhibitory projections [2, 3]. For example, those to the striatum are mirrored by GABAergic projections back to the midbrain and SN [2]. Lewy bodies, the pathological hallmarks of PD, appear in the brainstem before their emergence in basal ganglia (BG) and cortex according to postmortem evidence [4]. This motivates our study of GABAergic changes in the dopamine-depleted brainstem, as an important prerequisite for understanding the pathological and neurometabolic response that takes place within both the SN and the BG network. In the present study, based on our previous data [5, 6], we hypothesize that GABA+ levels (GABA + macromolecules + homocarnosine, as co-edited with MEGA-PRESS MRS [7]) are reduced in PD in the upper brainstem, where pathological changes precede those seen in the BG.Methods
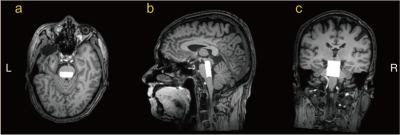
GABA+ levels were examined in 18 PD patients and 18 age- and sex-matched healthy controls (HCs). Structural MRI data were acquired using a T1-weighted MPRAGE sequence with sagittal acquisition, resolution 1.0 × 1.0 × 1.0 mm3; TR/TE 8.2/3.7 ms; flip angle = 8°, matrix = 256 × 256; field of view = 24 × 24 cm2. MEGA-PRESS [7] spectra were acquired from a 1.0 × 2.5 × 3.0 cm3 (AP×LR×HF) voxel centered on the midline of the upper brainstem [8], as shown in Figure 1. Sequence parameters were as follows: TR/TE = 2000/68 ms; 320 averages (and 8 water reference averages [9]); 2 kHz spectral width; 2048 data points; 14-ms sinc-Gaussian editing pulses applied at either 1.9 ppm (ON) or at 7.46 ppm (OFF) in interleaved fashion; minimum-phase excitation pulse bandwidth 2.2 kHz and slice-selective refocusing bandwidth 1.3 kHz; total scan time = 10 min 56 sec. Philips ‘MOIST’ water suppression (bandwidth 140 Hz) and Philips pencil-beam (PB-auto) shimming were used. GABA+-edited MRS was performed in 7.5-ml voxels in the upper brainstem, and the spectra were processed using the Gannet software. Differences in relaxation- and tissue-corrected GABA+ levels between the two groups were analyzed using independent t-test analysis.Results
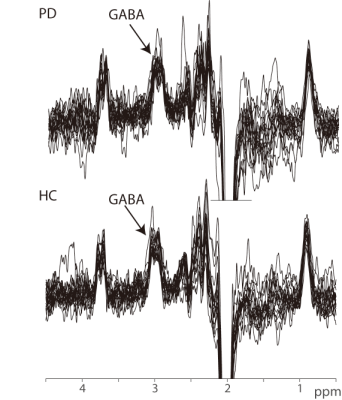
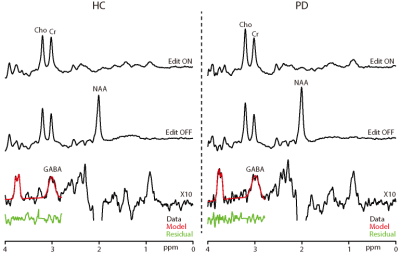
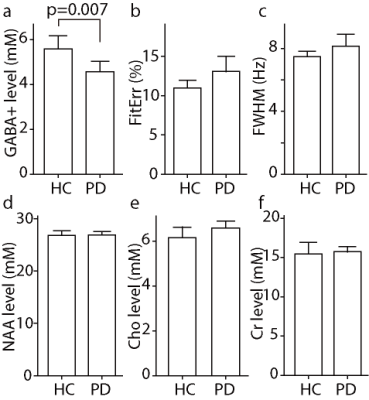
GABA+-edited spectra were successfully collected in the upper brainstem of all 36 participants; all spectra are shown in Figure 2, and those of one HC and one PD patient are compared in Figure 3 with ON and OFF subspectra to visualize Cr linewidth. GABA+ levels were significantly lower in the patients with PD (4.57±0.94 mM) than in the HC volunteers (5.89±1.16 mM; p < 0.05). Indices of data quality, such as the GABA+ fitting error and linewidth (both p > 0.05) were not significantly different between groups (as shown in Figure 4). Importantly, NAA, Cr, and Cho levels were not significantly different between PD and HC groups (NAA p = 0.82, Cr p = 0.72, and Cho p = 0.11) (also shown in Figure 4). No significant relationship was found between the Non-Motor Symptom Questionnaire (NMSQ) scores and GABA+ levels in PD patients (R = 0.104; p =0.192).Discussion
The main finding of this study was a significant reduction in the GABA+ levels in the upper brainstem regions of patients with PD compared with the HCs, consistent with our hypothesis. The primary pathology of PD is the degeneration of dopaminergic neurons, characterized by Lewy-body aggregation of misfolded α –synaptophysin, beginning from the lower brainstem (medulla oblongata) to the upper brainstem (pons, midbrain) and then to the limbic system, visceral motor system, and sensorimotor system [4]. Previous studies reported that PD pathology involves both dopaminergic neurons and GABAergic neurons located in the brainstem. The observed reduction in GABA+ levels would be consistent with either GABAergic neuronal loss in the brainstem, or changes in production, utilization or breakdown. Our GABA results are not well aligned with previous studies [10, 11] of brainstem GABA+ in PD, which reported significantly elevated levels. These studies differ from ours in terms of GABA quantification approach (linear combination modeling vs. editing), TE, voxel size and location (our ROI being larger and positioned higher in the brainstem). It is not clear whether the different results reflect opposite changes in different locations, or methodological differences. What is clear is that GABAergic neurons located in the brainstem play a role in regulating motor output, which appear to be impacted in PD.Conclusion
We confirmed the hypothesis that GABA+ levels are reduced in the upper brainstem of patients with PD. The results suggest that GABAergic dysfunction may be implicated in PD pathogenesis, suggesting a potential link between regional GABA+ levels and pathology progression in PD patients.Acknowledgements
No acknowledgement found.References
1. Samii, A., J.G. Nutt, and B.R. Ransom, Parkinson's disease. Lancet, 2004. 363(9423): p. 1783-93 DOI: 10.1016/S0140-6736(04)16305-8.
2. Lee, C.R. and J.M. Tepper, Basal ganglia control of substantia nigra dopaminergic neurons. J Neural Transm Suppl, 2009(73): p. 71-90 DOI: 10.1007/978-3-211-92660-4_6.
3. Tepper, J.M. and C.R. Lee, GABAergic control of substantia nigra dopaminergic neurons. Prog Brain Res, 2007. 160: p. 189-208 DOI: 10.1016/S0079-6123(06)60011-3.
4. Braak, H., et al., Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging, 2003. 24(2): p. 197-211 DOI: 10.1016/s0197-4580(02)00065-9.
5. Elmaki, E.E.A., et al., Examining alterations in GABA concentrations in the basal ganglia of patients with Parkinson's disease using MEGA-PRESS MRS. Jpn J Radiol, 2018. 36(3): p. 194-199 DOI: 10.1007/s11604-017-0714-z.
6. Gong, T., et al., Inhibitory motor dysfunction in parkinson's disease subtypes. J Magn Reson Imaging, 2018. 47(6): p. 1610-1615 DOI: 10.1002/jmri.25865.
7. Mescher, M., et al., Simultaneous in vivo spectral editing and water suppression. NMR Biomed, 1998. 11(6): p. 266-72 DOI: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j.
8. Song, Y., et al., Feasibility of Measuring GABA Levels in the Upper Brainstem in Healthy Volunteers Using Edited MRS. Frontiers in Psychiatry, 2020. 11(813) DOI: 10.3389/fpsyt.2020.00813.
9. Edden, R.A., et al., Prospective frequency correction for macromolecule-suppressed GABA editing at 3T. J Magn Reson Imaging, 2016. 44(6): p. 1474-1482 DOI: 10.1002/jmri.25304.
10. Emir, U.E., P.J. Tuite, and G. Oz, Elevated pontine and putamenal GABA levels in mild-moderate Parkinson disease detected by 7 tesla proton MRS. PLoS One, 2012. 7(1): p. e30918 DOI: 10.1371/journal.pone.0030918.
11. Oz, G., et al., Proton MRS of the unilateral substantia nigra in the human brain at 4 tesla: detection of high GABA concentrations. Magn Reson Med, 2006. 55(2): p. 296-301 DOI: 10.1002/mrm.20761.
Figures