3727
GABA and Susceptibility Changes in Striatum in Liver Cirrhosis: Preliminary Results1Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia, 2Institute of Radiology, University Medical Center Ljubljana, Ljubljana, Slovenia, 3Department of Gastroenterology and Hepatology, University Medical Center Ljubljana, Ljubljana, Slovenia, 4Russell H. Morgan Department of Radiology and Radiological Sciences, The John Hopkins University School of Medicine, Baltimore, MD, United States, 5M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 6Department of Neurology, Neuroimaging Research Unit, Medical University of Graz, Graz, Austria
Synopsis
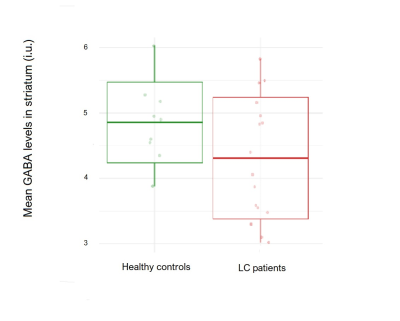
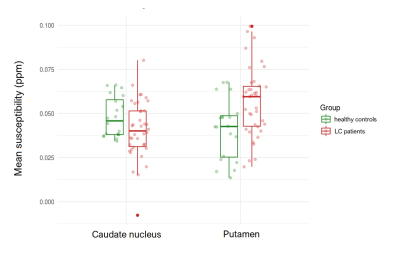
Liver cirrhosis (LC) is a worldwide public health problem. One of the complications of LC is hepatic encephalopathy which can present only as subtle cognitive impairment. Using advanced MR methods (MEGA-PRESS Spectroscopy and Quantitative Susceptibility Mapping), decreased striatal GABA levels, decreased susceptibility in caudate nucleus and increased susceptibility in putamen were demonstrated in LC patients compared to healthy controls. GABA levels and susceptibility in putamen correlated with the results of neuropsychological tests in the LC group compared to healthy controls.
Introduction
Liver cirrhosis (LC) is a worldwide public health problem that represents an important cause of death in adults. One of the complications of LC is hepatic encephalopathy (HE). In early stages, HE can present only as subtle cognitive impairment. In later stages, more evident clinical signs (e.g. motor impairment) are frequently observed in HE. Altered gamma-aminobutyric acid (GABA) levels1-4 and T2* MR changes in the striatum5 were previously demonstrated in HE patients. The striatum plays a critical role for the functioning of motor, cognitive and executive systems6,7; functions that are frequently affected in HE. This pilot study focuses on a more advanced assessment of the magnetic susceptibility and GABA levels in the striatum of LC patients and its relation to cognitive impairment.Methods
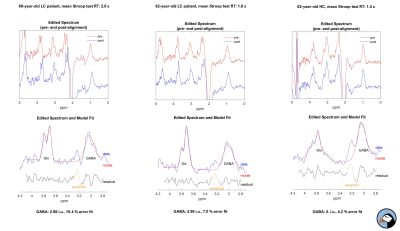
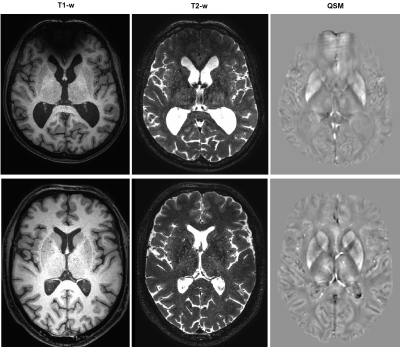
Twenty patients (six females, mean age: 61.7 +/- 9.5) with clinically diagnosed LC (all due to alcohol overconsumption) and ten (seven females, mean age: 58.2+/- 7.7) healthy subjects (HC) were included in the study. All subjects were right-handed (confirmed with Edinburgh Handedness Inventory). High-resolution T1- and T2-weighted images (voxel size 0.7x0.7x0.7 mm3) were acquired to place two 35x25x25 mm3 voxels for MEGA-PRESS8 MR spectroscopy in the left and right striatum. Data were acquired on a Philips Achieva 3 T scanner (Best, Netherlands). Data processing and quantitative analysis was performed in Gannet 3.0 (www.gabamrs.com). Mean GABA levels were calculated with respect to the tissue water signal and corrected for tissue composition. GABA levels from left and right striatum were averaged. Quantitative Susceptibility Mapping (QSM) was based on phase images of a 3D GRE sequence with multi-echo readout (voxel size 1.0x1.0x1.0 mm3, TR=35 ms, flip angle=15°, multiple TE: 11 ms, 16 ms, 21 ms and 26 ms). Phase unwrapping and dipole inversion was done with a total generalized variation approach9. The striatum and its anatomical subparts were segmented with Freesurfer 6.0.0 (surfer.nmr.mgh.harvard.edu). Mean susceptibility values in caudate nucleus and putamen were calculated in AFNI10 using 3dROIstats. Stroop test scores, psychometric hepatic encephalopathy score (PHES)11 and Mini-Mental State Exam (MMSE) scores were acquired. In the LC group, levels of venous blood ammonia were obtained on the day of the scan. T-tests for independent samples and Spearman correlation analyses were performed to investigate differences between groups and relationships with clinical scores. Statistical analysis was performed in R (www.r-project.org). Value of <0.05 was considered significant.Results
Spectra of three patients were excluded from the analysis due to motion artefacts. Mean GABA levels in the striatum were 4.24 +/- 0.95 i.u. and 4.9 +/- 0.63 i.u. in LC and HC group, respectively (p = .031). The mean susceptibility of caudate nucleus was 0.041 +/- 0.016 ppm and 0.048 +/- 0.011 ppm in LC and HC group, respectively (p = .029). Mean susceptibility in putamen was 0.057 +/- 0.020 ppm and 0.041 +/- 0.017 ppm in LC and HC group, respectively (p = .002). In the LC group, mean blood ammonia was 24.9 +/- 13.0 mmol/l. MMSE did not differ significantly between groups (29.9 +/- 0.32 vs. 29.1 +/- 1.5, p= .80). Mean response time of Stoop test was 4.24 +/- 0.89 seconds and 1.8 +/- 0.2 seconds in LC and HC group, respectively (p = .04). Mean time for completion of PHES was 521.7 +/- 172.6 seconds and 323.3 +/- 63.2 seconds in LC and HC group, respectively (p = .002). Negative correlations between the time needed for PHES completion, response time in Stroop test and striatal GABA levels were demonstrated (rho= -0.54, p = .005 and rho= 0.51, p = .012, respectively). Moreover, positive correlations were demonstrated between mean susceptibility in putamen, the time needed for PHES completion (rho= 0.58, p= .004) and response time in Stroop test (rho= 0.43, p= .013). No significant correlations were found between blood ammonia concentration, GABA levels and mean susceptibility in caudate nucleus or putamen.Discussion
In this pilot study, we found decreased striatal GABA levels in LC when compared to HC. Mechanisms of GABA regulation are complex. In presence of increased ammonia (a common feature of LC12), GABA neurotransmission is enhanced and astrocyte uptake GABA is reduced. Changes in GABA are linked to motor and cognitive deficits that are commonly found in HE. GABA is involved in osmotic regulation as well13. In LC, decreased susceptibility was demonstrated in caudate nucleus, whereas increased susceptibility was shown in putamen. Striatum, especially caudate nucleus, has an important role in cognition as well7. Decrease of susceptibility is most likely being caused by a decrease of iron concentration. Iron plays a crucial role in myelination14 and synthesis of neurotransmitters15. This preliminary study features a small sample size; continuation of recruitment and further stratification of patients in subgroups (according to cognitive impairment) may result in a model of predicting development of cognitive impairment in LC before the emergence of evident clinical signs.Conclusion
Using MEGA-PRESS and QSM, we demonstrated decreased striatal GABA levels, decreased susceptibility in caudate nucleus and increased susceptibility in putamen in LC compared to HC group. Striatal GABA levels and mean susceptibility of putamen correlated with the results of neuropsychological tests in the LC group compared to HC.Acknowledgements
This study was performed with grant P3-0019 from the Slovenian Research Agency (ARRS).References
1. Oeltzschner G, Butz M, Baumgarten TJ, et al. Low visual cortex GABA levels in hepatic encephalopathy: links to blood ammonia, critical flicker frequency, and brain osmolytes. Metab Brain Dis 2015;30:1429-1438
2. Behar KL, Rothman DL, Petersen KF, et al. Preliminary evidence of low cortical GABA levels in localized 1H-MR spectra of alcohol-dependent and hepatic encephalopathy patients. Am J Psychiatry 1999;156:952-954
3. Morley KC, Lagopoulos J, Logge W, et al. Brain GABA levels are reduced in alcoholic liver disease: A proton magnetic resonance spectroscopy study. Addict Biol 2018
4. Zöllner HJ, Oeltzschner G, Butz M, et al. J-edited Cerebral MR Spectroscopy in Patients with Hepatic Encephalopathy. ISMRM 27th Annual Meeting & Exhibition 2019; 2019
5. Liu JY, Ding J, Lin D, et al. T2* MRI of minimal hepatic encephalopathy and cognitive correlates in vivo. J Magn Reson Imaging 2013;37:179-186
6. Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. The Journal of neuroscience : the official journal of the Society for Neuroscience 2007;27:8161-8165
7. Provost JS, Hanganu A, Monchi O. Neuroimaging studies of the striatum in cognition Part I: healthy individuals. Front Syst Neurosci 2015;9:140
8. Mescher M, Merkle H, Kirsch J, et al. Simultaneous in vivo spectral editing and water suppression. NMR Biomed 1998;11:266-272
9. Langkammer C, Bredies K, Poser BA, et al. Fast quantitative susceptibility mapping using 3D EPI and total generalized variation. NeuroImage 2015;111:622-630
10. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162-173
11. Weissenborn K, Ennen JC, Schomerus H, et al. Neuropsychological characterization of hepatic encephalopathy. J Hepatol 2001;34:768-773
12. Khan A, Ayub M, Khan WM. Hyperammonemia Is Associated with Increasing Severity of Both Liver Cirrhosis and Hepatic Encephalopathy. Int J Hepatol 2016;2016:6741754
13. Ochoa-Sanchez R, Rose CF. Pathogenesis of Hepatic Encephalopathy in Chronic Liver Disease. J Clin Exp Hepatol 2018;8:262-271
14. Moller HE, Bossoni L, Connor JR, et al. Iron, Myelin, and the Brain: Neuroimaging Meets Neurobiology. Trends Neurosci 2019;42:384-401
15. Lu H, Chen J, Huang H, et al. Iron modulates the activity of monoamine oxidase B in SH-SY5Y cells. Biometals 2017;30:599-607
Figures