3725
Temporal correlation of functional connectivity and Choline in the monkey brain following ischemic stroke
Chun-Xia Li1, Frank Tong2, Doty Kempf1, Leonard Howell1, and Xiaodong Zhang1
1Yerkes Imaging Center, Yerkes National Primate Research Center, Emory University, Atlanta, GA, United States, 2Department of Radiology, Emory University, Atlanta, GA, United States
1Yerkes Imaging Center, Yerkes National Primate Research Center, Emory University, Atlanta, GA, United States, 2Department of Radiology, Emory University, Atlanta, GA, United States
Synopsis
Previous studies have suggested cerebral Choline (Cho) is a sensitive marker of acute stroke and could protect the tissue from ischemic injury. Also the relative connectivity (RelCon) could be a robust index to reveal the functional connectivity changes using resting state fMRI (rs-fMRI). The results indicated progressively increased RelCon in secondary somatosensory cortex (RelCon-S2) and a significant positive correlation between RelCon-S2 and relative cerebral Choline level (RelCho) from hyper-acute phase to 96 hours post stroke. The RelCon and RelCho combined detection might be an optimized and promising approach in management and prediction of stroke recovery.
Introduction
Previous studies have suggestted cerebral Choline (Cho) is sensitive to early stroke insult and could protect the tissue from ischemic injury [1, 2]. Also, the functional connectivity (FC) from resting state fMRI (rs-fMRI) has been used as one of neuroimaging markers to predict functional outcome and monitor the functional recovery after stroke [3]. The relative connectivity (RelCon) could be used as a robust index to reveal the FC changes in a longitudinal stroke study [4]. The stroke model of large animals like nonhuman primates (NHPs) shows superior advantage than rodents to improve the translational potential. The purpose of this study is to investigate the temporal relationship of the RelCon of secondary somatosensory cortex (S2) and corresponding cerebral Choline level in the monkey brain following stroke.Method
Permanent middle cerebral artery occlusion (MCAo) was induced in adult rhesus monkeys (n = 3, 10-19 years old) using minimally invasive interventional approach [1]. The rsfMRI and MR spectroscopy (MRS) data in monkeys were collected longitudinally on Day 0 (3-6 hours), Day 2(48 hours), and Day 4 (96 hours) post stroke on a Siemens 3T scanner with a 8-channel phased array knee coil. A gradient echo EPI sequence were used to acquire rsfMRI data (TR/TE=2190 ms/25ms, FOV=576mm×576mm, data matrix = 64 × 64, 430 volumes). T1, T2 and Diffusion-weighted images were acquired for image co-registration and lesion identification respectively. MR spectroscopy was collected using a chemical shift imaging pulse sequence with the parameters: TR/TE/NS = 1700ms/30ms/4, matrix = 16 × 16, voxel size = 4 × 4× 10 mm3 (Fig.1). The MRS data was analyzed with the LCModel software (Version 6.3-1L). The contralateral (Cho-CON) and ipsilateral (Cho-Ipsi) choline level (Fig. 1) around S2 region were selected to calculate relative Cho (RelCho), RelCho = Cho-Ipsi/ Cho-CON. SPSS 26.0 was used for statistical analysis.Data preprocessing (Slice timing correction, rigid body registration, regressing out signal in white matter and cerebrospinal fluid time series with a general linear model, temporal filtering with 0.009 Hz ~0.0237 Hz band-pass, spatial smooth by a Gaussian blur with 2.5-mm full width at half maximum) were performed using a script from AFNI (http://afni.nimh.nih.gov) for rsfMRI data analysis. Regions of interest (ROIs) of the contralateral (S2-Con) and ipsilateral (S2-Ipsi) S2 (Fig. 2) were defined based on the monkey brain atlas[2] and corresponding T1 weighted images. The averaged time courses of the S2-Con were used as seed signals for seed-based correlation analyses. Z transformation was applied to the individual correlation maps to show normalized correlation maps. RelCon-S2 = z score of S2-Ipsi / z score of S2-Con.
Analysis of variance (ANOVA) for repeated measures was performed to check the differences across serial time points for RelCon-S2 and RelCho. The Pearson correlation was applied between RelCon-S2 and RelCho on Day 0 (hyper acute stroke), Day 2, and Day 4 post occlusion.
Results
The rsfMRI results showed that the relative functional connectivity (RelCon-S2) progressively increased from hyper-acute phase to Day 4 post stroke (Fig.2 right). The RelCon-S2 at Day 4 was significantly higher than that in the hyper-acute phase though it was still much lower than the pre-surgery result (baseline) (Fig.3). The tendency of RelCho increase was seen following acute stroke, but no significant changes of RelCho were observed between different time points post stroke. However, the correlation result revealed a significant positive correlation between RelCon-S2 and RelCho from Day 0 to Day 4 post stroke (Fig.4).Discussion
Functional connectivity is a quantitative approach to quantify the functional deficit in experimental animals and stroke patients [3]. Recent studies suggest RelCon is a sensitive index due to its independent of image signal-to-noise ratio (SNR) [4] compared to the original z score in regular rsfMRI studies. The z score of S2-Ipsi in stroke monkeys showed evident trend of increase from Day 0 to Day 4 post occlusion but no significant difference could be reached. The present RelCon result suggested the recovery process of injured S2 started right after MCA occlusion as seen in the prior study [5]. Previous rodent studies suggested that choline could attenuate brain injury after stroke insult. Cho related medicine was recommended as an effective neuroprotective drug to protect brain from stroke as reported in previous studies [6] and preclinical trials [7, 8]. Therefore, it is motivating to evaluate the temporal correlation between the cerebral Choline level and functional deficit and recovery following acute stroke. Our preliminary results demonstrate RelCho is significant correlation with RelCon following stroke, indicating that the Cho probably involved in the recovery process.Conclusion
The preliminary results of a monkey model of stroke revealed that the RelCon and RelCho combined detection might be an optimized and promising approach in management and prediction of stroke recovery. As NHPs mimic most aspects of human and allow to conduct quantitative neurobehavior examination comparable to human, the monkey model of stroke and neuroimaging markers could facilitate the further investigation of the choline’s contribution to the functional recovery of stroke brain.Acknowledgements
No acknowledgement found.References
1. Karaszewski, B., et al., Brain choline concentration Early quantitative marker of ischemia and infarct expansion? Neurology, 2010. 75(10): p. 850-856. 2. Jin, X., et al., Brain protection against ischemic stroke using choline as a new molecular bypass treatment. Acta Pharmacologica Sinica, 2015. 36(12): p. 1416-1425. 3. Puig, J., et al., Resting-State Functional Connectivity Magnetic Resonance Imaging and Outcome After Acute Stroke. Stroke, 2018. 49(10): p. 2353-2360. 4. Golestani, A.M., et al., Longitudinal evaluation of resting-state FMRI after acute stroke with hemiparesis. Neurorehabil Neural Repair, 2013. 27(2): p. 153-63. 5. Zhang, X., et al., Temporal evolution of ischemic lesions in nonhuman primates: a diffusion and perfusion MRI study. PLoS One, 2015. 10(2): p. e0117290. 6. K.S. Saleem, N.K.L., A combined MRI and histology atlas of the rhesus monkey brain in stereotaxic coordinates. Academic Press, , London., 2007. 7. Golestani, A.M. and B.G. Goodyear, A resting-state connectivity metric independent of temporal signal-to-noise ratio and signal amplitude. Brain Connect, 2011. 1(2): p. 159-67. 8. Goodin, P., et al., Altered functional connectivity differs in stroke survivors with impaired touch sensation following left and right hemisphere lesions. Neuroimage Clin, 2018. 18: p. 342-355. 9. Maniega, S.M., et al., Choline and creatine are not reliable denominators for calculating metabolite ratios in acute ischemic stroke. Stroke, 2008. 39(9): p. 2467-2469. 10. Alvarez-Sabin, J. and G.C. Roman, The role of citicoline in neuroprotection and neurorepair in ischemic stroke. Brain Sci, 2013. 3(3): p. 1395-414. 11. Cho, H.J. and Y.J. Kim, Efficacy and safety of oral citicoline in acute ischemic stroke: drug surveillance study in 4,191 cases. Methods Find Exp Clin Pharmacol, 2009. 31(3): p. 171-6.Figures
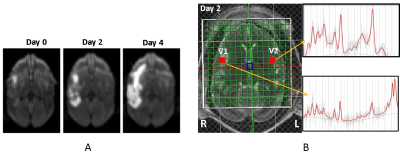
Figure 1. A) The diffusion-weighted images of a
stroke monkey brain demonstrated infarct
evolution on Day 0, 2, and 4 post
stroke (left). B) in vivo proton MR
spectra in the contralateral and ipsilateral voxels in the stroke monkey brain
2 days post stroke. V1, MR Spectrum of
contralateral voxel; V2, MR spectrum of ipsilateral area after MCA occlusion.
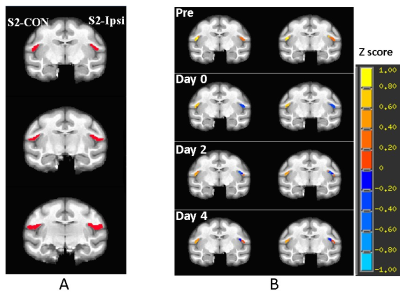
Figure 2. A) Illustration of the representative
slices of a stroke monkey brain with regions of interest of contralateral
secondary somatosensory cortex (S2)(S2-CON, seed for functional
connectivity analysis)
and the ipsilateral S2 (S2-Ipsi). B) representative correlation map of
S2 in a stroke monkey brain before stroke surgery
(Pre) and post surgery. p = 0.026 with 20 voxels as threshold.
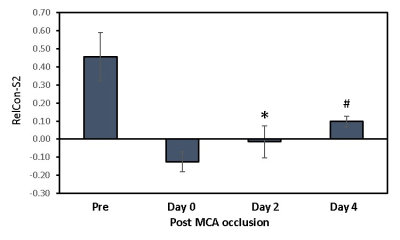
Figure 3.The
relative functional connectivity changes of
secondary somatosensory cortex (RelCon-S2) in monkey brains after MCA
occlusion. Pre, prescan
before MCA occlusion. *, p< 0.05 compared to prescan.
#, p<0.05 compared to the result on Day 0, 2, 4 post MCA occlusion. Error bar
indicates standard errors.
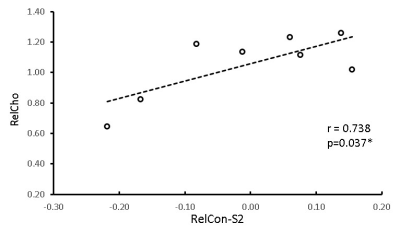
Figure 4. The
correlation between the
relative functional connectivity in secondary somatosensory cortex (RelCon-S2) and
relative
Choline (RelCho)
in
monkey
brains from Day 0 to Day 4 post
MCA occlusion.
*, p<0.05.