3724
Longitudinal Neurochemical Changes of Riluzole Therapy in Post-Traumatic Stress Disorder1Center for Clinical Spectroscopy, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, United States, 2Uniformed Services University of the Health Sciences, Bethesda, MD, United States, 3Office of Naval Research, United States Navy and Marine Corps, Alexandria, VA, United States
Synopsis
Active duty service members and veterans with post-traumatic stress disorder (PTSD) refractory to evidence-based therapy were randomized to receive either riluzole, a glutamate modulator, or placebo adjunctive to selective serotonin reuptake inhibitor therapy over an 8-week period. Subjects underwent 1H brain magnetic resonance spectroscopy scans pre- and post-treatment and were assessed longitudinally for PTSD symptomology using the Clinician-Administered PTSD Scale (CAPS). Subjects were stratified into improved and non-improved groups based on a decrease in CAPS score. Analyses on within- and between-group metabolite differences revealed that riluzole modulated the glutamate-glutamine cycle, improved neural energetics, and may have induced choline-linked neuroprotective inflammation.
Introduction
Post-traumatic stress disorder (PTSD) is a prevalent and debilitating condition that affects those who have been exposed to traumatic events. PTSD is typically treated with selective serotonin reuptake inhibitors (SSRI), but this therapy alone is not always effective.1 Glutamate, a major excitatory neurotransmitter, plays a critical role in the pathophysiology of PTSD.2 Riluzole inhibits glutamate release in presynaptic neurons3 and enhances astrocytic glutamate reuptake.4 The purpose of this study was to use magnetic resonance spectroscopy (MRS) to evaluate the efficacy of adjunctive riluzole therapy in modulating the neural concentrations and effects of glutamate in servicemembers diagnosed with combat-related PTSD.Methods
Subjects: Fifty-two active-duty servicemembers and Iraq/Afghanistan veterans being treated for PTSD with ongoing SSRI therapy were randomized into either a riluzole cohort or a placebo cohort. Subjects in the riluzole cohort received 100-200mg/day of riluzole in addition to their SSRI therapy for the duration of the study, depending on response to the medication.CAPS: Subjects were evaluated for PTSD symptomology using the Clinician-Administered PTSD Scale (CAPS)5 at baseline, mid-treatment (4 weeks), and post-treatment (8 weeks).
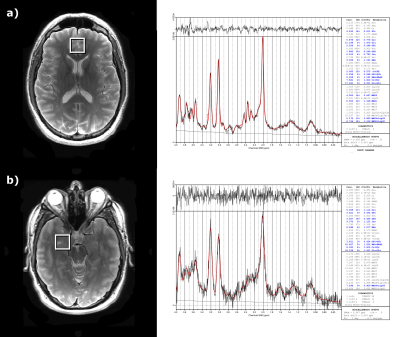
MRS Acquisition and Processing: 1H MRS scans were performed pre- and post-treatment using a 32 channel head coil on GE 3T MRI. Single-voxel spectroscopy (SVS) was acquired in the anterior cingulate cortex (ACC) and amygdala using the PRESS sequence with 35ms echo time (TE), 3000ms repetition time (TR), 5kHz bandwidth, and water suppression, with a corresponding unsuppressed water reference scan for scaling (Fig. 1). OpenMRSLab6 was used to perform coil combination, frequency correction, and phase correction on the acquired spectra. LCModel was used to perform eddy current correction and report metabolite concentrations.
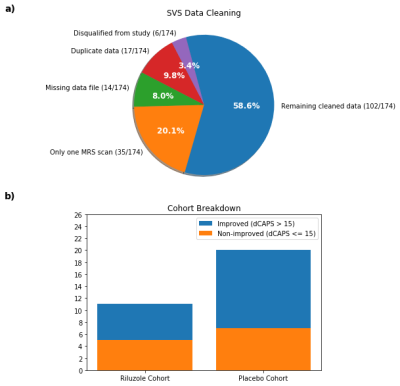
Data Cleaning and Statistical Analysis: Twenty-one subjects were either disqualified from the study or only completed one or fewer MRS scans and were omitted from data analysis (Fig. 2a), leaving 11 and 20 subjects in the riluzole and placebo cohorts, respectively (Fig. 2b). Statistical analysis was performed on metabolites with a mean Cramer-Rao lower bound (CRLB) lower than 12%: phosphocholine + glycerophosphocholine (tCho), creatine + phosphocreatine (tCr), N-acetylaspartate + N-acetylaspartylglutamate (tNAA), N-acetylaspartate (NAA), myo-inositol (Ins), glutamate + glutamine (Glx), and glutamate (Glu). Glutamine (Gln) was also included for its significance in riluzole’s metabolic pathway, but it had a mean CRLB of 44% so Gln findings must be interpreted with caution.
Analysis was performed with either the overall cohort, riluzole cohort alone, or the placebo cohort alone. Subjects with a greater than 15 point decrease in CAPS (dCAPS) were stratified into an improved group, while subjects with a dCAPS equal to or below 15 were stratified into a non-improved group.7 Metabolite changes over time were evaluated using the Wilcoxon signed-rank test and metabolite differences between groups were evaluated with the Mann-Whitney U test.
Results
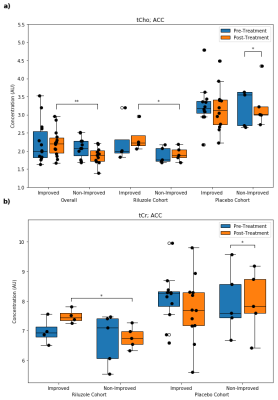
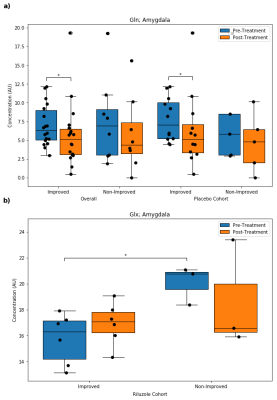
Anterior Cingulate Cortex: Overall, improved subjects had higher post-treatment tCho than non-improved subjects. In the riluzole cohort, improved subjects had higher post-treatment tCho and tCr than non-improved subjects. In the placebo cohort, improved subjects had lower pre-treatment Glu and higher post-treatment NAA, while non-improved subjects had decreased tCho and tCr over time (Fig. 3).Amygdala: Overall, improved subjects had higher pre-treatment tNAA than non-improved subjects and decreased Gln over time. In the riluzole cohort, improved subjects had lower pre-treatment Glx than non-improved subjects. In the placebo cohort, improved subjects had decreased Gln over time (Fig. 4).
Discussion
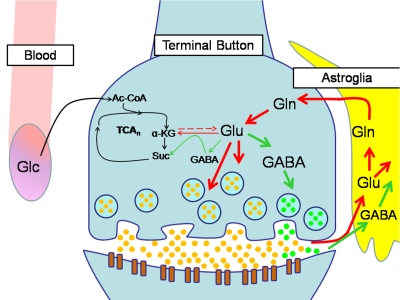
Previous studies investigating the neural effects of riluzole in other psychiatric disorders have shown that riluzole may decrease overall levels of Glx8 and alter Glu-Gln cycling (Fig. 5).9 The lower pre-treatment amygdala Glx in the improved riluzole cohort and other significant Glu/Gln findings in ACC and amygdala may arise from inherent differences between the two groups, but could also indicate a relationship between lower Glx levels and sensitivity to symptomatic improvement that is moderated by riluzole’s effects on Glu-Gln cycling.MRS studies comparing PTSD to healthy controls have shown increased neural tCho in PTSD.10 tCho has also been shown to be linked to neuroinflammation.11 The elevated post-treatment tCho levels in the improved riluzole cohort suggest that choline may serve a neuroprotective role rather than a neurodegenerative one in PTSD.12 Furthermore, the same trend is observed in overall improved subjects, suggesting that choline could serve as a biomarker for long-term improvements in PTSD symptomatology. Finally, the higher post-treatment tCr levels in the improved riluzole cohort indicate that neural energetics are improved in responders to riluzole whereas non-responders did not show this improvement.
Conclusion
While the findings of this study may be confounded by the adjunctive SSRI therapy and low Glu/Gln specificity at 3T,13 they do suggest that riluzole modulates the Glu-Gln cycle in responders and point to a tCho-linked neuroprotective inflammatory response in PTSD. These findings provide the motivation for a larger future study to further investigate these findings with additional MRS-based randomization criteria. Higher-field MRI scanners could also be used to investigate the Glu-Gln cycle in PTSD more closely.Acknowledgements
The ideas presented in this work are those of the authors and do not necessarily reflect those of the Department of Defense or the Henry Jackson Foundation.References
1. Alexander W. (2012). Pharmacotherapy for Post-traumatic Stress Disorder In Combat Veterans: Focus on Antidepressants and Atypical Antipsychotic Agents. P & T : a peer-reviewed journal for formulary management, 37(1), 32–38.
2. Averill, L. A., Purohit, P., Averill, C. L., Boesl, M. A., Krystal, J. H., & Abdallah, C. G. (2017). Glutamate dysregulation and glutamatergic therapeutics for PTSD: Evidence from human studies. Neuroscience letters, 649, 147–155. https://doi.org/10.1016/j.neulet.2016.11.064
3. Chéramy, A., Barbeito, L., Godeheu, G., & Glowinski, J. (1992). Riluzole inhibits the release of glutamate in the caudate nucleus of the cat in vivo. Neuroscience letters, 147(2), 209–212. https://doi.org/10.1016/0304-3940(92)90597-z
4. Frizzo, M. E., Dall'Onder, L. P., Dalcin, K. B., & Souza, D. O. (2004). Riluzole enhances glutamate uptake in rat astrocyte cultures. Cellular and molecular neurobiology, 24(1), 123–128. https://doi.org/10.1023/b:cemn.0000012717.37839.07
5. Weathers, F. W., Bovin, M. J., Lee, D. J., Sloan, D. M., Schnurr, P. P., Kaloupek, D. G., Keane, T. M., & Marx, B. P. (2018). The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychological assessment, 30(3), 383–395. https://doi.org/10.1037/pas0000486
6. Rowland, B. C., Sreepada, L., Jiang, S., & Lin, A. P. (2017). OpenMRSLab: An open‐source software repository for magnetic resonance spectroscopy data analysis tools.
7. Weathers, F. W., Keane, T. M., & Davidson, J. R. (2001). Clinician-administered PTSD scale: a review of the first ten years of research. Depression and anxiety, 13(3), 132–156. https://doi.org/10.1002/da.1029
8. Pillinger, T., Rogdaki, M., McCutcheon, R. A., Hathway, P., Egerton, A., & Howes, O. D. (2019). Altered glutamatergic response and functional connectivity in treatment resistant schizophrenia: the effect of riluzole and therapeutic implications. Psychopharmacology, 236(7), 1985–1997. https://doi.org/10.1007/s00213-019-5188-5
9. Brennan, B. P., Hudson, J. I., Jensen, J. E., McCarthy, J., Roberts, J. L., Prescot, A. P., Cohen, B. M., Pope, H. G., Jr, Renshaw, P. F., & Ongür, D. (2010). Rapid enhancement of glutamatergic neurotransmission in bipolar depression following treatment with riluzole. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 35(3), 834–846. https://doi.org/10.1038/npp.2009.191
10. Seedat, S., Videen, J. S., Kennedy, C. M., & Stein, M. B. (2005). Single voxel proton magnetic resonance spectroscopy in women with and without intimate partner violence-related posttraumatic stress disorder. Psychiatry research, 139(3), 249–258. https://doi.org/10.1016/j.pscychresns.2005.06.001
11. Jung, C., Ichesco, E., Ratai, E.M., Gonzalez, R.G., Burdo, T, Loggia, M.L., Harris, R.E., Napadow, V. (2020). Magnetic resonance imaging of neuroinflammation in chronic pain: a role for astrogliosis? Pain. 2020 Jul;161(7):1555-1564. doi: 10.1097/j.pain.0000000000001815. PMID: 31990749; PMCID: PMC7305954.
12. Sochocka, M., Diniz, B. S., & Leszek, J. (2017). Inflammatory Response in the CNS: Friend or Foe?. Molecular neurobiology, 54(10), 8071–8089. https://doi.org/10.1007/s12035-016-0297-1
13. Govindaraju, V., Young, K., & Maudsley, A. A. (2000). Proton NMR chemical shifts and coupling constants for brain metabolites. NMR in biomedicine, 13(3), 129–153. https://doi.org/10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v
Figures